Fig. 1.
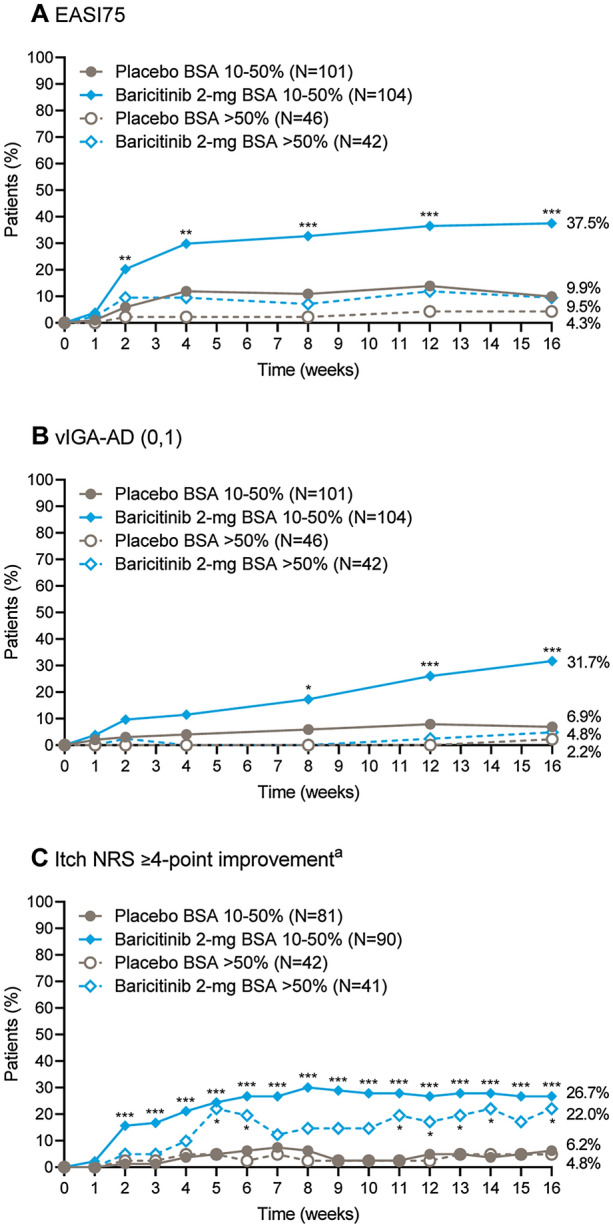
Clinical response by baseline BSA category. Proportion of patients achieving A a 75% improvement in total EASI score, B a vIGA-AD score of 0 or 1, or C a ≥ 4-point improvement in the Itch NRS response over time, among patients who had a baseline BSA of 10–50% or > 50%. aAssessed for patients with a baseline Itch NRS score ≥ 4. *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001 for baricitinib 2 mg compared with placebo. BSA body surface area, EASI75 75% improvement in Eczema Area and Severity Index score, NRS Numeric Rating Scale, vIGA-AD (0,1) validated Investigator Global Assessment for Atopic Dermatitis score of 0 or 1
