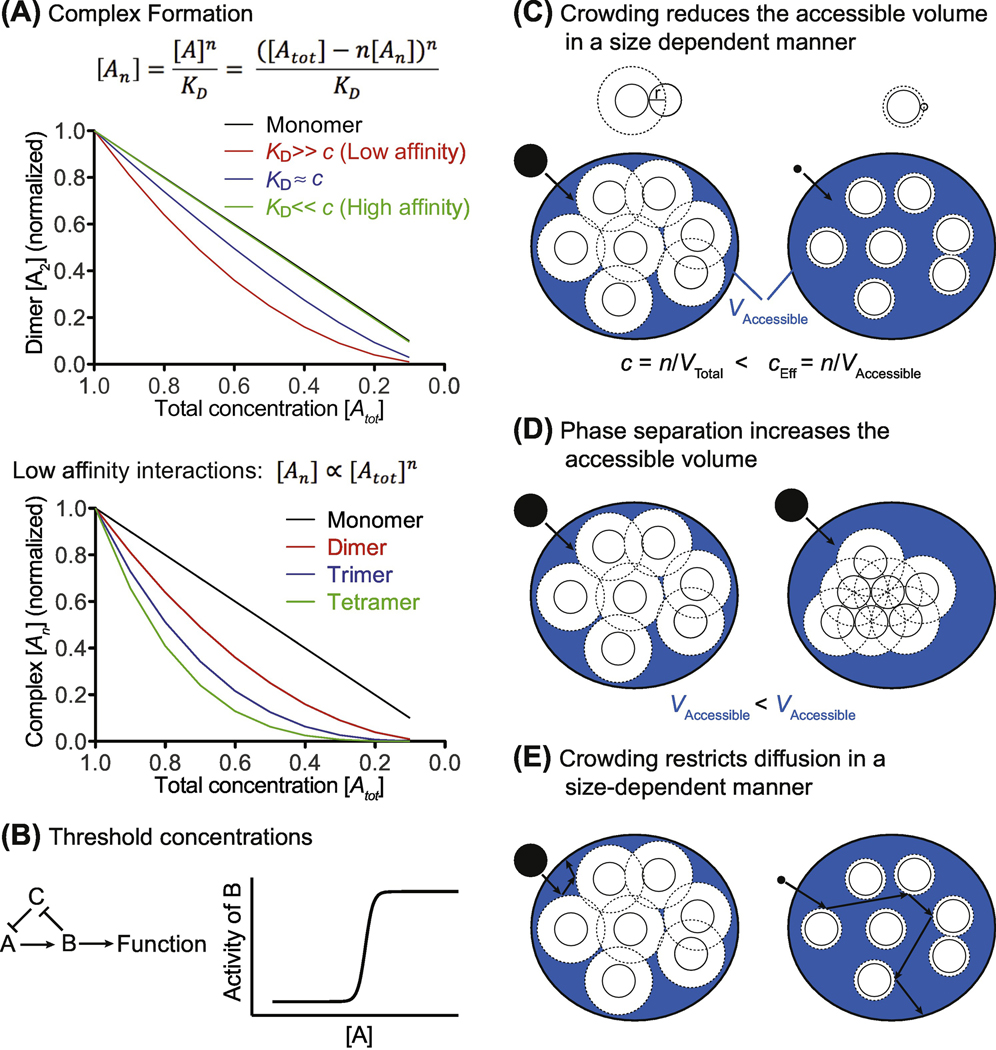
Figure 2: Theoretical impact of alterations in cytoplasm density on biological processes.
(A) Simulation of multi subunit protein complex concentration [An] as a function of changing total protein concentration [Atot] as described by the law of mass action. For simplicity we are considering homomultimers, but the conclusions also apply to heteromeric complexes. KD dissociation constant. Top: Dimers [A2] that bind with low affinity are more sensitive to changes in total protein concentration than dimers that bind with high affinity. Bottom: For low affinity interactions, the concentration of total protein is much larger than the concentration of the complex [Atot] >> [An]. The concentration of monomeric protein is therefore nearly the same as the total protein concentration [A] ≈ [Atot]. The concentration of multimers with weakly interacting subunits is therefore proportional to the product of the concentrations of all subunits [An] α [Atot]n.
(B) Feedback regulation can generate sharp transitions between different cellular states. The components A, B and C represent a feedback loop. Activity of A is inhibited by C. If A surpasses a threshold concentration, it initiates the feedback loop by activating B, which in turn inactivates C, allowing for full activation of A and B. Cytoplasm density can influence whether or not key regulatory proteins reach threshold concentrations that trigger such transitions.
(C) Large macromolecules, such as ribosomes (white circles), occupy up to 20% of the cytoplasm volume, reducing the accessible volume (blue, VAccessible) for other macromolecules (black filled circles). As a result, the effective concentration of a molecule (cEff) in the cytoplasm is higher than the total concentration (c) inside the cell, and biochemical reactions are accelerated by the excluded volume effect. The center of macromolecules (black filled circles) can only approach other macromolecules (white circles) up to a distance of half its diameter (dashed line). Large molecules (left cartoon) are therefore excluded from a larger volume than small molecules (right cartoon). One consequence of this phenomenon is that compact protein conformations are entropically favored in a crowded environment. Crowding thus facilitates protein folding. n=number of molecules, V=volume.
(D) Molecular crowding promotes the generation of phase-separated condensates (right cartoon) because this reduces the excluded cell volume and increases the accessible volume for other molecules. This state is therefore entropically favored.
(E) Molecular crowding restricts lateral diffusion. Large molecules (left cartoon) are more affected by this than small molecules (right cartoon), because their center cannot move as closely to other particles for steric reasons.