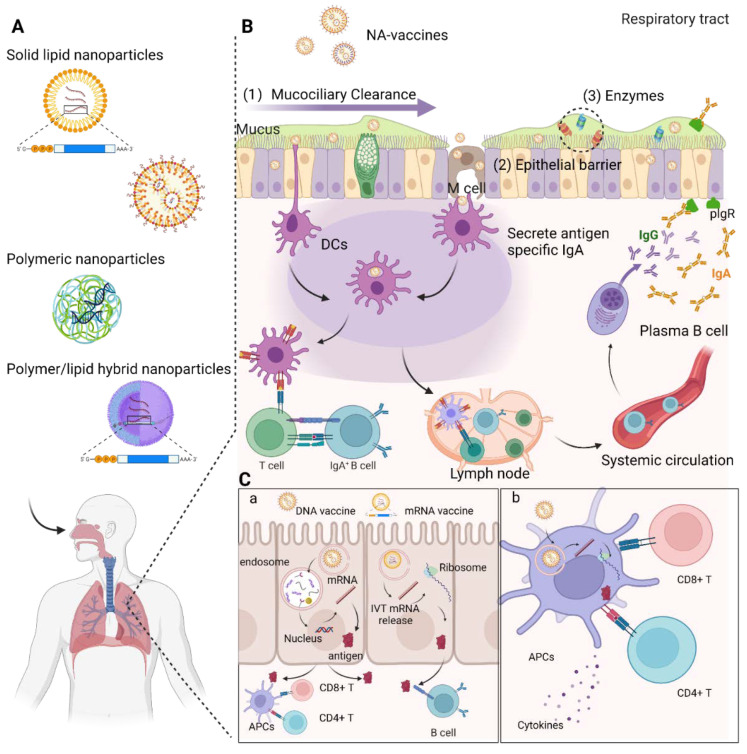
Figure 1.
Overview of nucleic acid-based (NA-) vaccines administrated via the respiratory tract using nanotechnologies. (A) Schematic view of different nanoparticles used for intranasal and pulmonary vaccinations. (B) Physical and biological barriers at the airway mucosal site and mechanism of immune responses in the respiratory tract mediated by mucosal-associated lymphoid tissues (MALTs). NA-vaccines transcytose from the mucus layer into the epithelial tissues by microfold cells (M cells) or passively diffuse through epithelial cell junctions. Other NA-vaccines are captured and internalized by APCs, such as DCs, from their extension through epithelial junctions. APCs that have been transfected with genetic antigens migrate to the nearest lymph node to activate T cells and B cells. Activated B cells proliferate in the lymph node and enter the systemic circulation to the mucosal effector sites. B cells locally differentiate into antibody-secreting plasma cells to produce IgA dimers. IgA dimers are secreted via pIgR at the mucosal surface. Antigen-specific systemic IgG is also produced. (C) NA-vaccines are taken up by epithelial cells (a), and pathogen-derived antigens are then transcribed and translated from plasmid DNA or IVT mRNA and secreted into the extracellular space, where they can be taken up by professional APCs such as DCs. (b). APCs then present antigens to naïve T cells for activation and differentiation, promoting humoral and cell-mediated immune responses against the encoded antigen.