Table 4.
Summary of polymer-modification improvement strategies.
| No. | Modification Routes | Improvement Strategies/Reaction Schemes |
|---|---|---|
| 1 | Incorporation of CO2-philic groups into the polymer to improve polarity towards CO2. Oxygen functionalities can improve sorption properties. The interaction can be via hydrogen bonding or electrostatic. | Incorporation of various Lewis bases/pendant polar groups such as ether, hydroxyl, carboxylic and carbonyl oxygen promotes physical interaction with CO2 (electronegativity) due to higher polarity, thus producing higher CO2 solubility. It can produce extra hydrogen bonding that further alters the pore size of the membranes, resulting in higher activation energy for CO2 and CH4. The incorporation of micropores from the incorporation of polar groups have enhanced the permeability of CO2.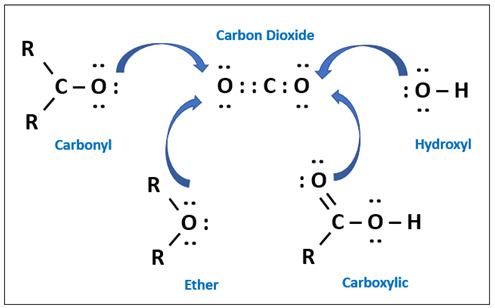
|
| 2 | Incorporation of bulky groups to improve chain stiffness and limit chain packing |
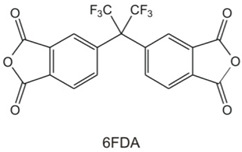 Bulky hexafluoro-substituted carbon (–C(CF3)2) groups in dianhydride structure of 6FDA-based PIs increased chain stiffness, hence increasing selectivity while inhibiting chain packing since the bulky group can serve as a molecular spacer which can increase the permeability and reduce aging. 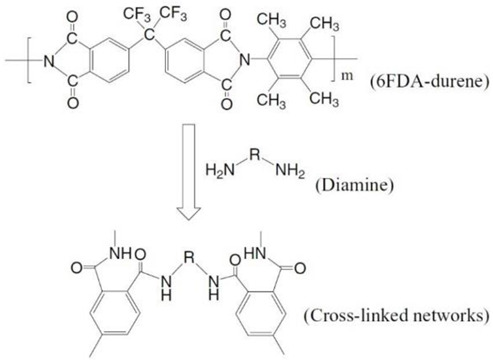 Bulky diamines help disrupt chain packing while increasing free volume. This contributes to the enhancement in permeability, while the aromatic rings are responsible for chain rigidity for better selectivity [25] and aging resistance. Amines strongly and selectively bind the CO2 via chemisorption leading to higher heat of adsorption [86]. Bulky tetra-o-isopropyl and naphthalene groups are introduced to membranes, resulting in high FFV values and enhanced polymeric backbone rigidity. The disturbed chain packing leads to high gas permeabilities [87] and aging resistance. Rigid, bulky and diamond-like structure Adamantane is grafted into the membrane main chain and side chain through a simple acyl chloride-substitution reaction to adjust the chain packing. D-spacing of the membranes could be finely tuned by adjusting the mole ratio of grafted adamantane moiety. The resultant membrane CO2 permeability is enhanced significantly [88]. |
| 3 | Functionalization using bromination to increase FFV and thus permeability. |
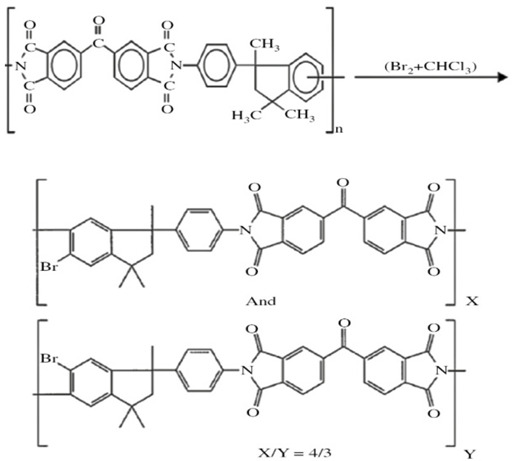 Brominated Matrimid 5218 membranes were much more permeable due to the higher FFV of the brominated membranes [89]. |
| 4 | Sulfonation to improve rigidity and FFV |
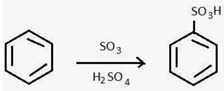 Sulfonation reaction successfully enhanced the microvoids, promoted interchain hydrogen bonding by the SO3H group and boosted the rigidity. As the degree of sulfonation increased, gas permeability increased by 3.2-fold [83] and improved aging resistance. |
| 5 | Lithiation to improve solubility, FFV and chain rigidity |
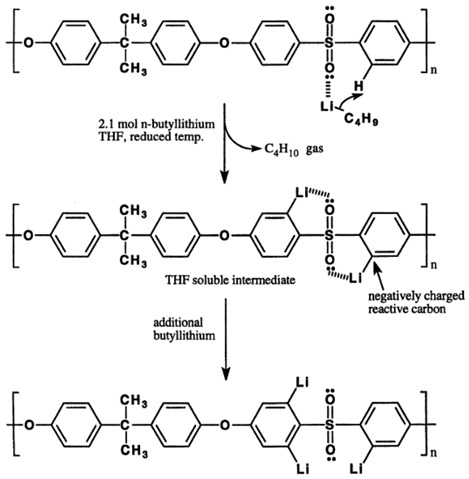 Lithiation is performed in PSF due to the availability of strong electron-withdrawing effect and capability of the lone pairs of electrons on the sulfone oxygen atoms to combine with the lithiation agent. Butyllithium substitutes the ortho-sulfone hydrogen atoms with lithium atoms so that the aromatic carbon atoms to which lithium is attached nominally have a negative charge, which can improve its performance and enhance attraction towards CO2 [90]. |
| 6 | Esterification by diols or polyols |
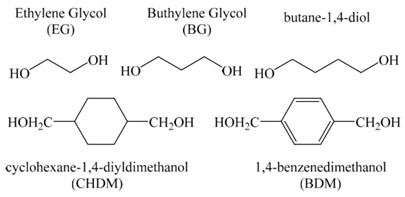 Common diol cross-linkers can be introduced through carboxylic acid or sulfonic acid groups to form ester bonds, linking the two polymer chains [3]. Esterification, which involves the reaction between acid and alcohol, serves as a function to create limited rotational ability [91]. |
