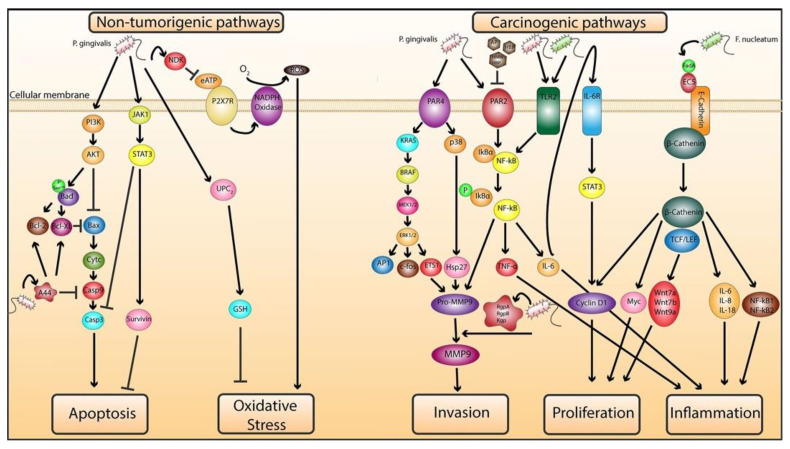
Figure 1.
Proposed molecular mechanisms of P. gingivalis and F. nucleatum-mediated tumorigenesis in tumorous and nontumorous cells. Nontumorigenic pathways: (left). P. gingivalis promotes antiapoptosis by activating JAK1/STAT3 and PI3K/AKT prosurvival signaling pathways to inhibit caspase3 (casp3) and activate survivin. P. gingivalis-mediated activation of PI3K/AKT leads to the phosphorylation of Bad at serine residue 136 and its activation results in its dissociation from antiapoptotic Bcl2 and Bcl-XL proteins, which enhances the antiapoptotic effect of the Bcl2 family reflected by the inhibition of apoptotic protein Bax. Furthermore, Bcl-2 and Bcl-XL are upregulated by P. gingivalis gigipain’s adhesin peptide A44, which also inhibits Casp9 activation at early stages. Inhibition of Bax impedes cytochrome c (cytc) release from the mitochondria and blocks the cleavage of casp9 and subsequent activation of effector caspase3, which obstructs the mitochondrial intrinsic apoptotic pathway. P. gingivalis inhibits P2X7 receptor (P2X7R)/NADPH oxidase-mediated ROS production and subsequent apoptosis by blocking extracellular ATP (eATP) ligation to P2X7R through its ATP-scavenging enzyme, nucleoside diphosphate kinase (NDK). Additionally, P. gingivalis induces antioxidant responses by increasing glutathione (GSH) levels intracellularly possibly by upregulating the uncoupling protein 2 (UCP2). Carcinogenic pathways: (right). P. gingivalis activates protease-activated receptor 4 (PAR4), which in turn activates the ERK1/2/Ets1 and p38/Hsp27 pathways resulting in pro-MMP9 production. P. gingivalis’s activation of PAR2 can mediate pro-MMP9 production via the NF-kB pathway. The cleaved active form of pro-MMP9 is MMP9. Metalloproteinase (MMP) families are involved in ECM and basement membrane degradation and enhance invasion in neoplastic cells. P. gingivalis through its virulent factor, a cysteine protease termed gingipain, can cleave pro-MMP9 into its active form MMP9. Gingipains consist of arginine-specific protease A (RgpA) and B (RgpB) and a lysine-specific protease (Kgp), which are responsible for the cleavage of pro-MMP9 into MMP9. Apple polyphenol (AP), hop bract polyphenol (HBP) and high-molecular weight HBP (HMW-HBP) are polyphenols that can inhibit the proteolytic activity of gingipains and can inhibit the PAR2/NF-kB release of pro-MMP9. Thus, P. gingivalis promotes invasion through gingipain-mediated activation of MMP9. F. nucleatum’s virulent factor FadA binds to the extracellular domain 5 (EC5) of E-cadherin receptor and activates β-catenin that stimulate cyclin D1 and Myc upregulation and binds to T-cell factor/lymphoid enhancer factor (TCF/LEF) to stimulate the secretion of Wnt7a, Wnt7b and Wnt9a, all of which promote cellular growth and proliferation. β-catenin enhances the production of cytokines IL-6, IL-8 and IL-18 and NF-kB1/2 and promotes inflammation, an optimal microenvironment for the prosperity of cancerous cells. F. nucleatum and P. gingivalis can activate toll-like receptor 2 (TLR2) and mediate TNF-α and IL-6 cytokine production via the TLR2-NF-kB pathway. IL-6 stimulates the activation of the IL-6 receptor (IL-6R), which in turn activates STAT3, known to ultimately upregulate the production of cyclin D1 and promote cellular proliferation.