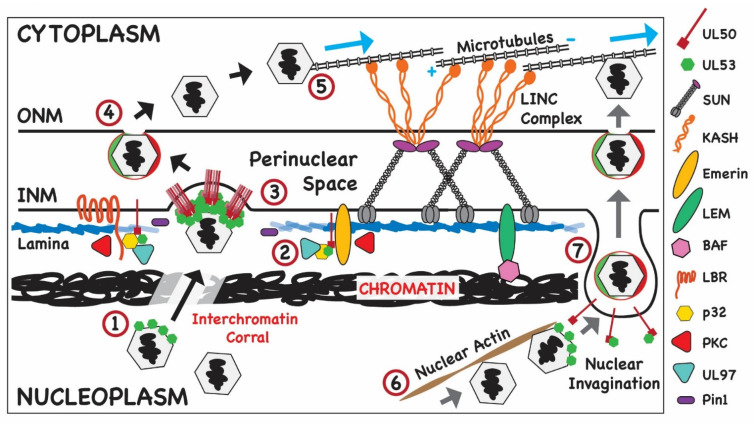
Figure 3.
Modification of nuclear structures that allow HCMV nuclear egress. The structure of nuclear membrane described in Figure 2 is shown with modification of nuclear structure that could act as barriers for egress of HCMV capsids. (1) Newly formed capsids decorated with pUL53 are shown transiting chromatin through an interchromatin corral; (2) disruption of the nuclear lamina by host cell proteins such as Pin 1 and the viral protein kinase, UL97; (3) loss of this barrier followed by heterodimerization of the core NEC of HCMV (pUL53 and pUL50) at the INM promotes primary envelopment of capsids at the INM. (4) Following primary envelopment at the INM and transit through the PNS, the enveloped capsid then fuses with the ONM and undergoes deenvelopment, releasing the particle into the cytoplasm; (5) following acquisition of the inner tegument proteins, the tegumented capsid is transported on MT to sites of cytoplasmic assembly. (6) Alternatively, the capsid could be transported by nuclear actin to sites of budding from the INM; (7) following interactions between accessory viral proteins and pUL53 of the NEC, heterodimerization of pUL53 and pUL50 leading to primary envelopment at a nuclear invagination generated through disruption of the nuclear lamina as described above. Following primary envelopment, transit through the PNS space and fusion with the ONM, the capsid is released from ONM and trafficked to sites of virus assembly in the cytoplasm as described above.