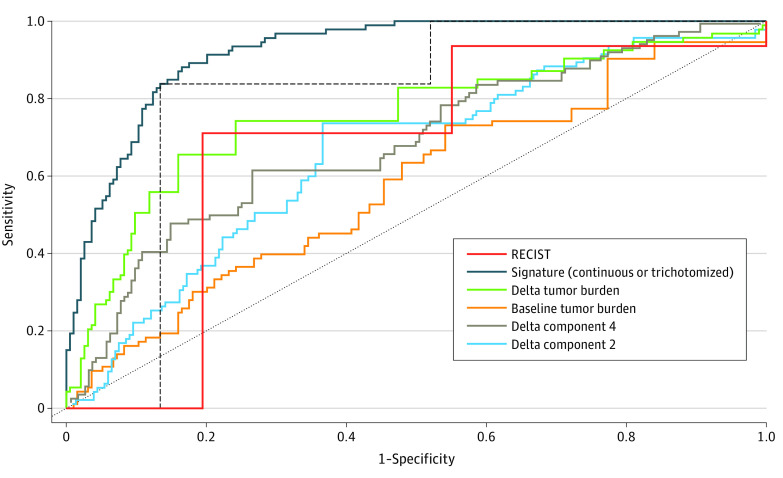
Figure 2. Prediction of Overall Survival (OS) Using Radiomics Signature vs Response Evaluation Criteria in Solid Tumors 1.1 (RECIST 1.1) in the Validation Set.
The regulatory end point defined progression-free survival and overall response rate. In the pembrolizumab validation set (n = 287), trichotomized RECIST 1.1 response classified patients at 3 months as having a complete or partial response (93 of 287 [32.4%]), stable disease (90 of 287, [31.3%]), and progressive disease (104 of 287 [36.2%]). The signature was trichotomized from best to worse predicted prognosis using the same distribution as RECIST 1.1 (classified patients into 3 groups, including 32.4%, 31.3%, and 36.2% of patients as cutoffs to divide the signature results into top, middle, and bottom groups). The area under the time-dependent receiver operating characteristics curve for prediction of survival status at 6 months posttreatment landmark was 0.92 (95% CI, 0.89-0.95) for the continuous signature, 0.89 (95% CI, 0.86-0.92) for the trichotomized signature, and 0.80 (95% CI, 0.74-0.84) the trichotomized RECIST 1.1.