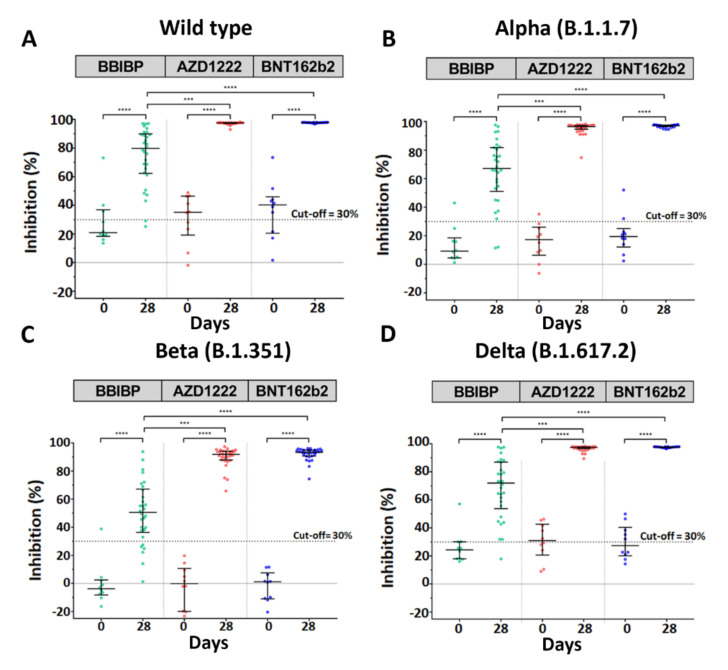
Figure 3.
Neutralization activities against wild-type and SARS-CoV-2 variants measured by surrogate virus neutralization test (sVNT). The serum samples obtained from participants who received two completed doses of the inactivated vaccine, CoronaVac; followed by the inactivated vaccine, BBIBP (green); the viral vector vaccine, AZD1222 (red); or the mRNA vaccine, BNT162b2 (blue), at 3–4 months after the first dose were compared. The neutralizing activities against SARS-CoV-2 Wide-type (A), Alpha (B.1.1.7) (B), Beta (B.1.351) (C), and Delta (B.1.617.2) (D) were shown. Lines represent medians with interquartile ranges (IQR); ns indicates no significant difference; p-value < 0.001 (***), 0.0001 (****).