Fig. 5.
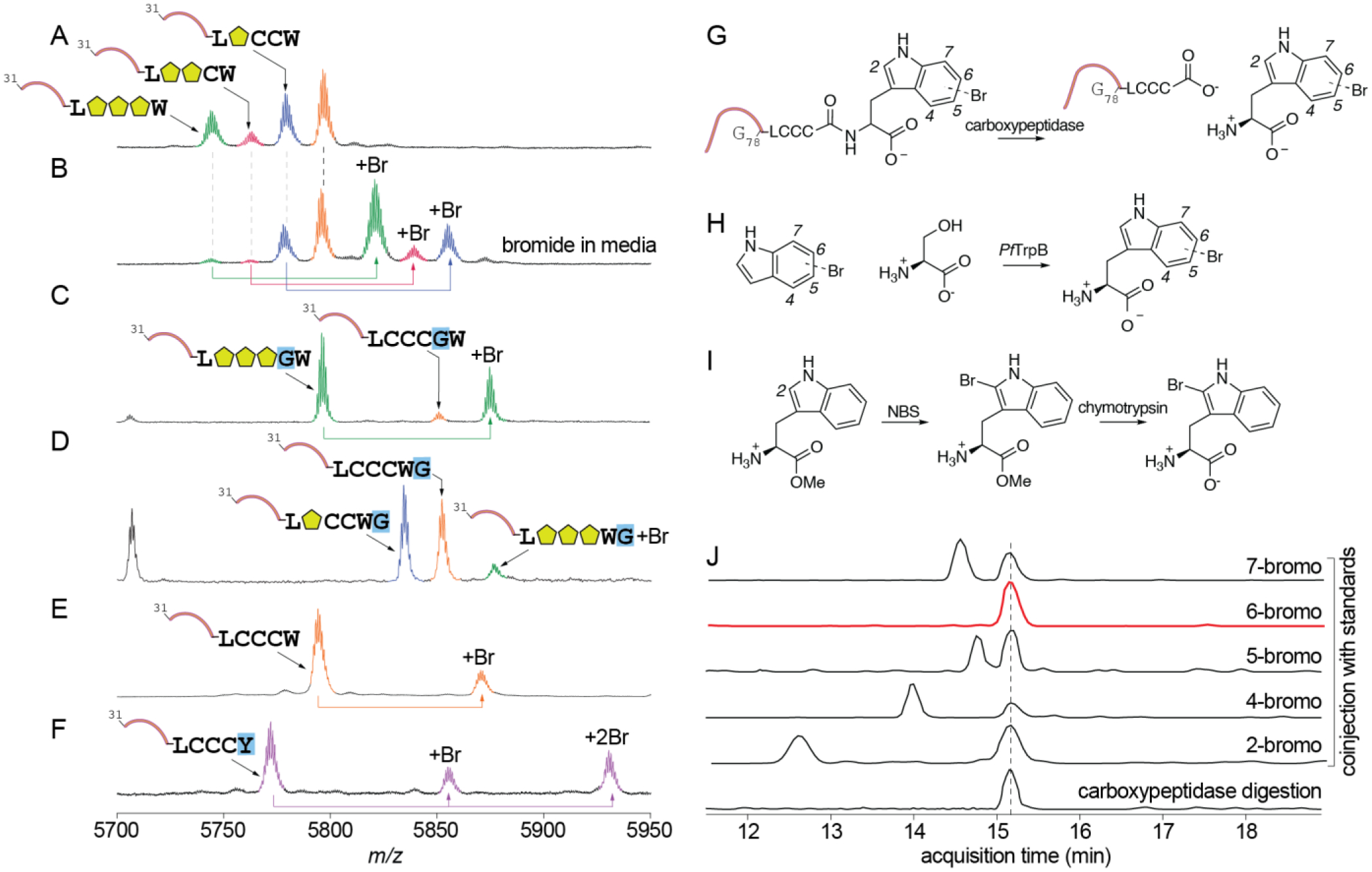
Activity of RiPP brominase 1SrpI. (A) No halogenated products were observed upon coexpression of 1sprE with 1srpC and 1srpI when culture media lacked bromide. (B) Bromination of the mono-, di-, and tri-cyclodehydrated 1SrpE was observed when culture media was supplemented with bromide. Processing of the (C) 1SrpE-LCCCGW and (D) 1SrpE-LCCCWG by 1SrpC and 1SrpE to yield cyclodehydrated-brominated products. (E) When gene 1srpC was omitted from the co-expression experiment, bromination of the unmodified 1SrpE peptide was observed. (F) Mono- and di-bromination was observed when the C-terminal tryptophan residue was modified to tyrosine. (G) To determine the regiospecificity of tryptophan halogenation by 1SrpI, gene 1srpE was co-expressed with 1srpI and the purified 1SrpE peptide product was digested with carboxypeptidase to excise the C-terminal monobrominated tryptophan residue. (H) Standards for 4-, 5-, 6-, and 7-bromotryptophan were generated by condensation of L-serine with bromoindoles using tryptophan synthase PfTrpB. (I) Scheme for synthesis of the 2-bromotryptophan standard. NBS: N-bromosuccinimide. (J) The carboxypeptidase digestion reaction was co-injected with 2-, 4-, 5-, 6-, and 7-bromotryptophan standards and the presence of monobrominated tryptophan was monitored by LCMS using extracted ion chromatograms for m/z 284.14 ± 0.1 Da. The 1SrpE-excised bromotryptophan residue from 1SrpE coeluted with the 6-bromotryptophan standard.
