Table 2.
A comparison of the substituted 2-mercaptoquinazolin-4(3H)-ones yields1 obtained with selected green methods with literature yields.
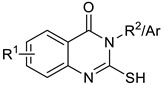
| |||||||
|---|---|---|---|---|---|---|---|
| Compounds | R 1 | Ar/R 2 | Ystirring (%) 2 | YMW (%) 3 | YUS 4 (%) | YLit. (%) | Mp (°C) |
| 6a | H | Me | 42 | 39 | 17 | 46 [48]; 68 [33]; 60 [49] | 264–265 |
| 6b | H | Et | 40 | 18 | 36 | 72 [33]; 95 [50] | 255 |
| 6c | H | Allyl | 24 | 15 | 21 | 35 [33] | 206–208 |
| 6d | H | Ph | 63 | 22 | 34 | 35 [48]; 90 [33]; 92 [50]; 73 (82) [51]; 61 [49]; 75 (60) [52] | 304–305 |
| 6e | H | Bn | 62 | 41 | 64 | 50 [48]; 83 [53] | 248–250 |
| 6f | H | 4-MePh | 27 | 47 | 38 | 94 [50]; 79 (78) [51]; 88 [54], 75 (62) [52] | 312–313 |
| 6g | H | 4-FPh | 48 | 32 | 25 | 88 [3]; 88 [54] | 336–337 |
| 6h | H | 4-ClPh | 55 | 49 | 41 | 75 [33]; 89 [50]; 94 [51] | 331–332 |
| 6i | H | 4-BrPh | 66 | 24 | 58 | 84 [55]; 70 [52] | 330–331 |
| 6j | H | 3-OMePh | 64 | 21 | 57 | 91 [54]; 65 (50) [52] | 285 |
| 6k | H | 3-ClPh | 41 | 12 | 25 | 86 [54] | 300–301 |
| 7a | 6-I | Me | 31 | 20 | 40 | 73 [33] | 307–308 |
| 7b | 6-I | Et | 25 | 14 | 18 | 10 [33] | 290–292 |
| 7c | 6-I | Allyl | 22 | 13 | 21 | 20 [33] | 234–235 |
| 7d | 6-I | Ph | 32 | 16 | 22 | 91 [33]; 70 [52] | 350–352 |
| 7e | 6-I | Bn | 45 | 10 | 28 | 352 | |
| 7f | 6-I | 4-MePh | 49 | 29 | 47 | 350–351 | |
| 7g | 6-I | 4-FPh | 43 | 22 | 18 | 349–350 | |
| 7h | 6-I | 4-ClPh | 39 | 31 | 38 | 75 [29] | 337–339 |
| 7i | 6-I | 4-BrPh | 49 | 30 | 51 | 355–357 | |
| 7j | 6-I | 3-OMePh | 53 | 25 | 58 | 314–315 | |
| 7k | 6-I | 3-ClPh | 50 | 25 | 44 | 313–315 | |
| 8a | 6-Br | Me | 21 | 14 | 23 | 64 [49] | 280–281 |
| 8b | 6-Br | Et | 25 | 13 | 17 | 243–244 | |
| 8c | 6-Br | Allyl | 40 | 14 | 40 | 242–243 | |
| 8d | 6-Br | Ph | 36 | 13 | 19 | 63 [49]; 75 [52] | 351–353 |
| 8e | 6-Br | Bn | 58 | 27 | 60 | 244 | |
| 8f | 6-Br | 4-MePh | 76 | 31 | 41 | 60 [56] | 341–342 |
| 8g | 6-Br | 4-FPh | 57 | 19 | 28 | 354–355 | |
| 8h | 6-Br | 4-ClPh | 62 | 27 | 48 | 344–346 | |
| 8i | 6-Br | 4-BrPh | 65 | 33 | 56 | 349–350 | |
| 8j | 6-Br | 3-OMePh | 49 | 28 | 30 | 312–313 | |
| 8k | 6-Br | 3-ClPh | 57 | 18 | 33 | 305–307 | |
| 9a | 7-Cl | Me | 58 | 19 | 18 | 327–328 | |
| 9b | 7-Cl | Et | 22 | 15 | 21 | 265 | |
| 9c | 7-Cl | Allyl | 47 | 13 | 26 | 248–249 | |
| 9d | 7-Cl | Ph | 67 | 19 | 36 | 71 (90) [51] | 313–314 |
| 9e | 7-Cl | Bn | 26 | 17 | 34 | 270–272 | |
| 9f | 7-Cl | 4-MePh | 42 | 13 | 24 | 73 [51] | 307–309 |
| 9g | 7-Cl | 4-FPh | 34 | 18 | 18 | 314–315 | |
| 9h | 7-Cl | 4-ClPh | 50 | 14 | 27 | 76 [53] | 302–303 |
| 9i | 7-Cl | 4-BrPh | 50 | 16 | 38 | 320–322 | |
| 9j | 7-Cl | 3-OMePh | 40 | 25 | 44 | 256–257 | |
| 9k | 7-Cl | 3-ClPh | 39 | 19 | 54 | 248–249 | |
| 10a | 6,8-(Cl)2 | Me | 36 | 15 | 41 | 246–247 | |
| 10b | 6,8-(Cl)2 | Et | 19 | 119 | 15 | 184 | |
| 10c | 6,8-(Cl)2 | Allyl | 21 | 23 | 30 | 179 | |
| 10d | 6,8-(Cl)2 | Ph | 20 | 18 | 19 | 65 [52] | 283–285 |
| 10e | 6,8-(Cl)2 | Bn | 51 | 14 | 27 | 206–208 | |
| 10f | 6,8-(Cl)2 | 4-MePh | 25 | 27 | 40 | 70 [52] | 244 |
| 10g | 6,8-(Cl)2 | 4-FPh | 33 | 22 | 24 | 268–269 | |
| 10h | 6,8-(Cl)2 | 4-ClPh | 34 | 30 | 30 | 259–260 | |
| 10i | 6,8-(Cl)2 | 4-BrPh | 60 | 37 | 48 | 280–282 | |
| 10j | 6,8-(Cl)2 | 3-OMePh | 33 | 14 | 24 | 219–220 | |
| 10k | 6,8-(Cl)2 | 3-ClPh | 38 | 24 | 43 | 214–216 | |
1 The yields were calculated based on the anthranilic acid for the product after precipitation; 2 ChCl:urea (1:2), 80 °C, 1 h; 3 ChCl:urea (1:2), 1800 W, 80 °C, 1 h; 4 ChCl:urea (1:2), 50 W, 80 °C, 1 h.
