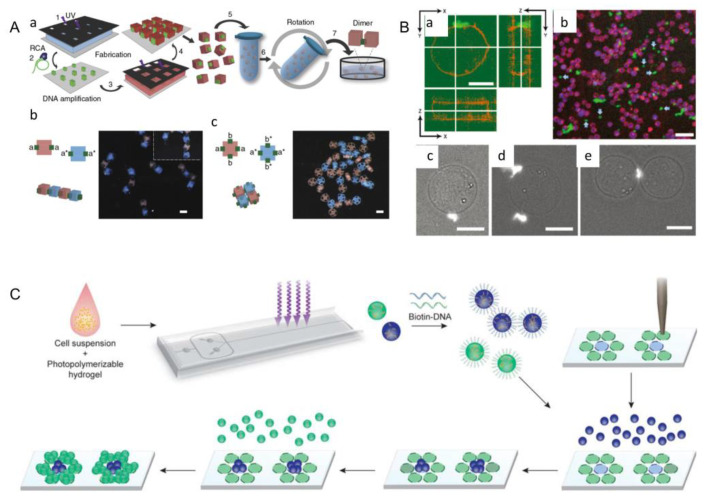
Figure 10.
(A) The process of the DNA-assisted assembly. (Reproduced with permission from the Reference [95]). (a) The hydrogels that surround the cells are labeled with different single strands of DNA. According to the principle of base complementary pairing, the hydrogel dimer is formed under the action of hydrogen bond between corresponding bases. Four different DNA strands are used to decorate the four sides of the cubic hydrogel units, so that the specific connection could be realized in four different directions, and the chain and mesh assembly structures are finally obtained. (B) Attachment of fluorescent DNA hexagonal arrays through streptavidin (STV) to biotinylated Jurkat cells. (a) Confocal cross-section of fluorescent-labeled DNA-biotin-STV array binding to biotinylated Jurkat (leukemia) cells. Jurkat cells appear orange on the surface. (b) Confocal microscopy field of fluorescent-labeled DNA-biotin array binding to biotinylated Jurkat cells. Unbound arrays are represented by light blue arrows. The DNA array is green and the Jurkat cell surface is red; Jurkat’s cytoplasm is blue. (c–e) fluorescence micrograph DNA-biotin array binds to Jurkat-biotin cells. (Reproduced with permission from the Reference [94]). (C) Using DNA’s good molecular recognition ability to achieve rapid template assembly for multiple microtissue types. The cells are injected into a photopolymerizable hydrogel pre-polymer high flux microfluidic encapsulation device. Droplets of the cell-prepolymer mixture are exposed to ultraviolet light on the chip to form streptavidin-containing microstructures, which are then terminated with biotin oligonucleotides. Encoded microtissues containing different cell types are seeded onto a DNA microarray template, which guide the microtissues to bind to specific points on the surface of the template to obtain cell-bearing microtissues. (Reproduced with permission from the Reference [94]).