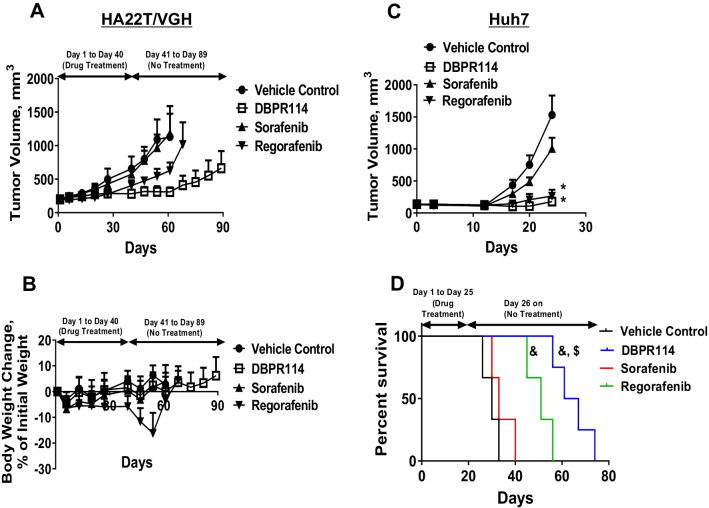
Fig. 7.
Effect of DBPR114 and regorafenib on sorafenib-refractory and sorafenib-acquired resistant human HCC xenograft tumors. A and B Tumor growth curves and body weight changes from baseline (%) for the control and treated HA22T/VGH xenograft tumors. C Tumor growth curves for the control and treated sorafenib-acquired resistant Huh7 xenograft tumors. D Survival in the control and treated sorafenib-acquired resistant Huh7 xenograft tumor groups. DBPR114 (40 mg/kg) was administered once a week intravenously for 6 weeks for the HA22T/VGH xenograft tumors and 3 weeks for the sorafenib-acquired resistant Huh7 xenograft tumors. Sorafenib and regorafenib were administered at 30 mg/kg once a day, 5 days per week by oral gavage for 40 days for the HA22T/VGH xenograft tumors and 25 days for the Huh7 xenograft tumors. Mean ± SEM, n = 8 mice per group for both xenograft tumors. *p < 0.05 vs. vehicle control measured using one-way ANOVA and Bonferroni posttest comparison. &p < 0.01 vs. control, $p < 0.05 vs. regorafenib, measured using Mantel–Cox test