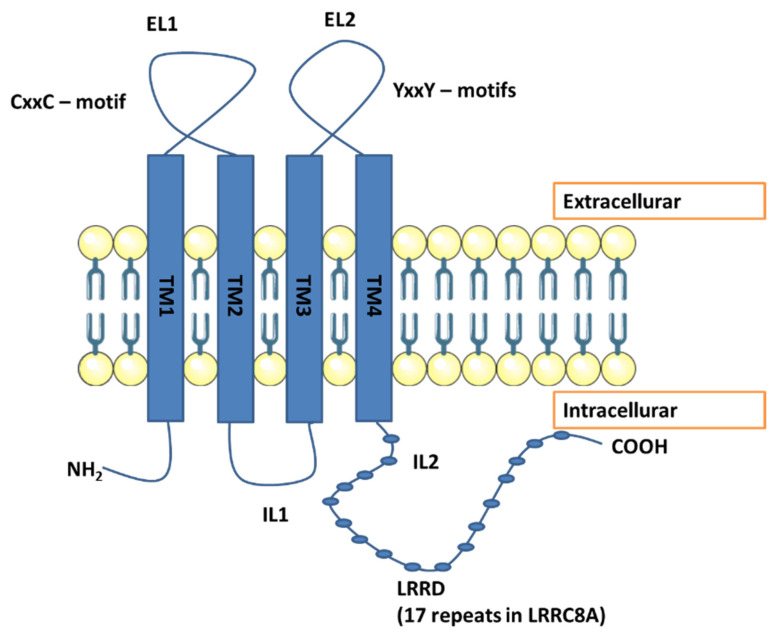
Figure 1.
The figure shows the structure of the LRRC8 subunits. High-resolution cryo-electron microscopy enabled to visualization of the structure of LRRC8 subunits 145,146. Studies have shown the presence of 4 permeable transmembrane helices (TM) located at the amino terminus and the carboxyl terminus, which contain 17 leucine-rich repeats. Moreover, the TM1-TM2 (EL1) and TM3-TM4 (EL2) domains are extracellularly located, and the amino and carboxyl terminus and the TM2-TM3 (IL1) and IL2 domain to be located intracellularly. According to the study of the LRRC8A protein sequence and knowledge of the domains interacting with cisplatin, the existence of cisplatin-interacting domains within LRRC8A, such as methionine in cellular and transmembrane segments, and metal-binding CxxC/YxxY motifs (letters denote specific amino acids, x is a random amino acid) [27].