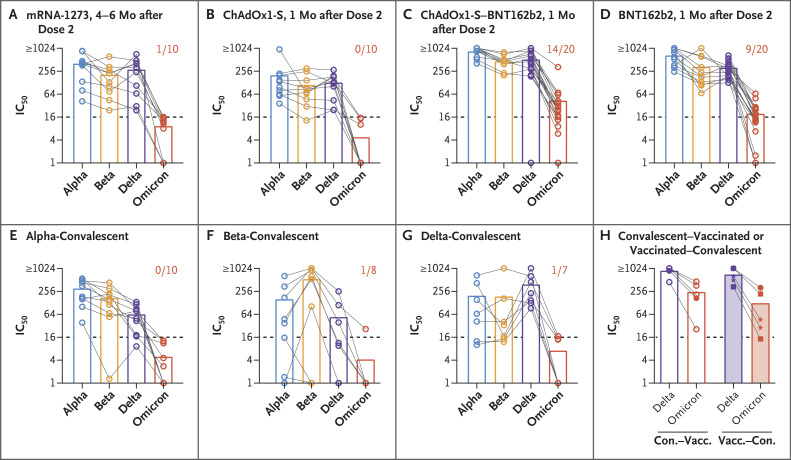
Figure 1. Neutralization of the B.1.1.529 (Omicron) Variant as Compared with Other Variants of Concern.
Serum samples were obtained from participants who had received two doses of the mRNA-1273 vaccine (Panel A), two doses of the ChAdOx1-S vaccine (Panel B), heterologous ChAdOx1-S–BNT162b2 vaccination (Panel C), or two doses of the BNT162b2 vaccine (Panel D) or who had recovered from infection (i.e., convalescent) with the B.1.1.7 (alpha) variant (Panel E), the B.1.351 (beta) variant (Panel F), or the B.1.617.2 (delta) variant (Panel G). Samples were analyzed for 50% neutralization titers (IC50) against the alpha (blue), beta (orange), delta (purple), and omicron (red) variants. Bars indicate means, and symbols individual serum samples. Samples from the same participant are connected by lines. The dashed line in each panel indicates the limit of detection. The numbers in Panels A through G indicate the proportion of serum samples that were positive (>1:16) for the omicron variant. Serum samples from participants who had been infected and were subsequently vaccinated (convalescent–vaccinated; open bars) or who had been vaccinated and subsequently had breakthrough infection (vaccinated–convalescent; shaded bars) were analyzed for IC50 against the delta and omicron variants (Panel H). In the left part of Panel H (convalescent–vaccinated), open circles indicate participants who received a single dose of the BNT162b2 vaccine after infection, and closed circles those who received two doses of the BNT162b2 vaccine; in the right part (vaccinated–convalescent), closed circles indicate participants who had been vaccinated with two doses of the BNT162b2 vaccine before infection, stars those who had been vaccinated with two doses of the ChAdOx1-S vaccine, and squares those who had been vaccinated with two doses of the mRNA-1273 vaccine.