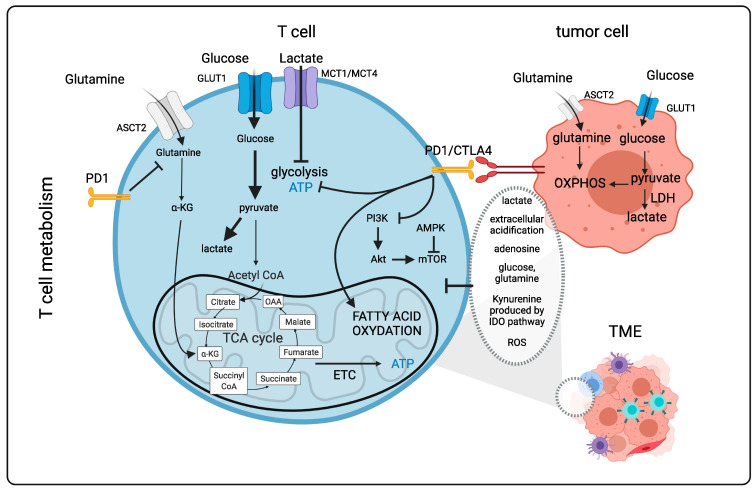
Figure 1.
T cell metabolism in the tumor microenvironment. Naïve T cells rely mainly on oxidative metabolism. Following activation with an antigen, T cells switch to a glycolytic metabolism by activation of the mTOR pathway. This metabolic program supports effector T cell functions. If antigen stimulation persists long term, such as in the tumor environment, inhibitory receptors such as PD1 and CTLA4 can rewire T cell metabolism by reducing glycolysis and glutaminolysis, which weakens effector functions. Other factors in the TME contributing to the exhausted state of T cells include low levels of oxygen, low levels of tryptophan metabolized into kynurenine by IDO, low levels of arginine, high levels of lactate and resulting acidification and strong competition of T cells with cancer cells for glucose and glutamine. PD1: Programmed cell death 1; CTLA4: cytotoxic T-lymphocyte-associated protein 4; ASCT2: ASC amino-acid transporter 2; GLUT1: glucose transporter 1; OAA: Oxaloacetate; α-KG: α-Ketoglutarate; AMPK: Adenosine monophosphate kiinase; LDH: Lactate dehydrogenase; TME: Tumor microenvironment; Akt: Protein kinase B; mTOR: mammalian target of rapamycin; ATP: Adenosine triphosphate; ROS: Reactive oxygen species; PI3K: Phosphoinositide 3-kinase; IDO: Indoleamine-pyrrole 2,3-dioxygenase; ETC: Electron transport chain; TCA: Tricarboxylic acid; CoA: Coenzyme A. Figure generated with Biorender.com (accessed on 15 November 2021).