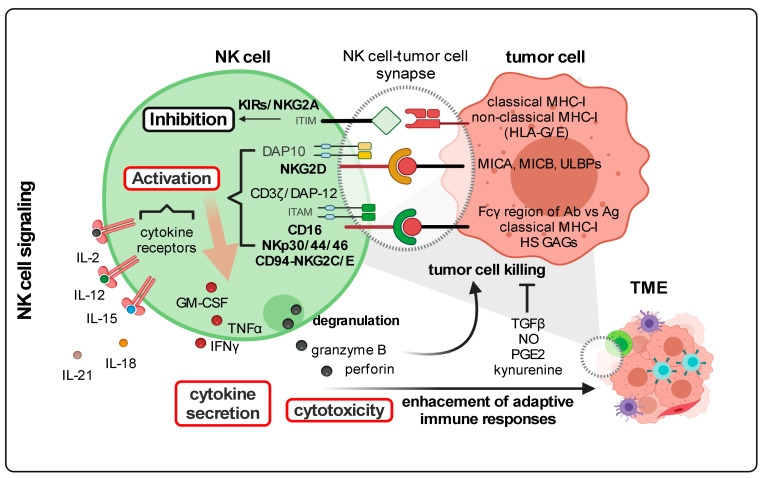
Figure 4.
Simplified overview of the human NK cell-tumor cell synapse. Tumor cells signal to inhibitory and activating receptors expressed on NK cells. The binding of KIRs and NKG2A to MHC-I molecules leads to NK cell inhibition; engagement of NKG2D, NKp30/44/46, NKG2C/E and CD16; and drives the activation of NK cells. Upon receptor engagement, inhibitory signaling is mediated by ITIM motifs. Activating signaling can be ITAM-dependent and ITAM-independent. Activating receptors commonly form complexes with adaptor molecules such as DAP10 and DAP12 that trigger the activating signaling cascade. In addition to NK cell activation mediated by receptor engagement, cytokines also stimulate NK cells. Total NK cell activation is characterized by the production of inflammatory cytokines and degranulation, which leads to the release of cytolytic granules containing perforin and granzyme B. Lytic enzymes released at the synapse between NK cells and tumor cells warrant tumor cell clearance. Cytokines secreted by NK cells strengthen adaptive immune responses depending on several soluble factors within the TME such as TGFβ, NO, PGE2 and L-kynurenine that are secreted by tumor and stromal cells, limit the killing of tumor cells by NK cells. KIRs: Killer cell Immunoglobulin-like receptors; DAP: DNAX-activating protein; ITIM: Immunoreceptor tyrosine-based inhibitory motifs; ITAM: Immunoreceptor tyrosine-based activation Motifs; MHC-I: Major Histocompatibility Complex I; HLA: Human Leukocyte Antigen; MICA, MICB: MHC class I chain-related protein A and B; ULBPs: UL16 binding proteins; HS GAGs: Heparan Sulfate Glycosaminoglycans; Ab: Antibody; Ag: Antigen; GM-CSF: Granulocyte monocyte-colony stimulating fFactor; TNFα: Tumor cecrosis factor α; IFNγ: Interferon γ; IL: interleukin; TGFβ: Transforming growth factor β; NO: Nitric oxide; PGE2: Prostaglandin E2; TME: Tumor microenvironment. Figure generated with Biorender.com (accessed on 15 November 2021).