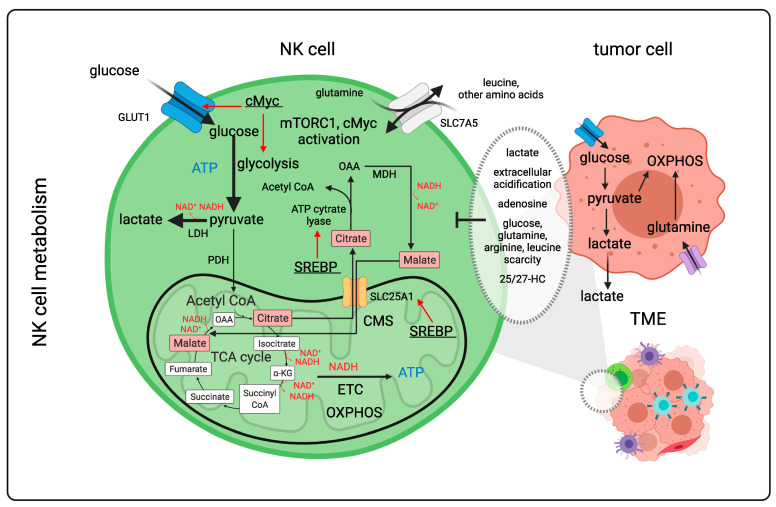
Figure 5.
NK cell metabolism upon activation and challenges within the TME. NK cells rely on glucose to sustain glycolysis and OXPHOS for the production of energy. The citrate–malate shuttle between mitochondrial citrate and cytosolic malate provides sufficient reducing equivalents in the cytoplasm to sustain glycolysis and glycolytic ATP production while generating lactate. The citrate–malate shuttle also generates NADH molecules in the mitochondria apart from the ones that are generated by the tricarboxylic acid during OXPHOS. NADH molecules are oxidized by the electron transport chain to produce ATP. Upon NK cell activation, SREBPs transcriptionally controls the expression of the malate–citrate antiporter SLC25A1 and the ATP citrate lyase. In activated NK cells, the glucose transporter Glut1 is upregulated as well as the aa transporter SLC7A5. The exchange of intracellular glutamine for other aa, such as leucine through SLC7A5, increases the intracellular aa availability that is required to activate mTORC1 and enhance cMyc expression. cMyc transcriptionally controls glycolysis as well as the expression of the glucose transporter GLUT1. NK cells at the synapse with tumor cells and within the tumor microenvironment encounter extracellular acidification. This is mediated by a higher content of lactate secreted by tumor cells, inhibitory metabolites such as adenosine, limited availability of glucose and aa such as glutamine, arginine and leucine, as well as soluble inhibitors of SREBPs such as 25/27-HC. LDH, lactate dehydrogenase; PHD, pyruvate dehydrogenase; TCA, tricarboxylic acid; OAA, oxalacetate; α-KG, α-ketoglutarate; ETC, electron transport chain; OXPHOS, oxidative phosphorylation; CMS, citrate–malate shuttle; SREBPs, sterol regulatory element-binding proteins; mTORC1, mammalian target of rapamycin complex 1; MDH, malate dehydrogenase; 25/27-HC, hydroxycholesterol; TME, tumor microenvironment. aa, amino acid. Figure generated by Biorender.com (accessed on 15 November 2021).