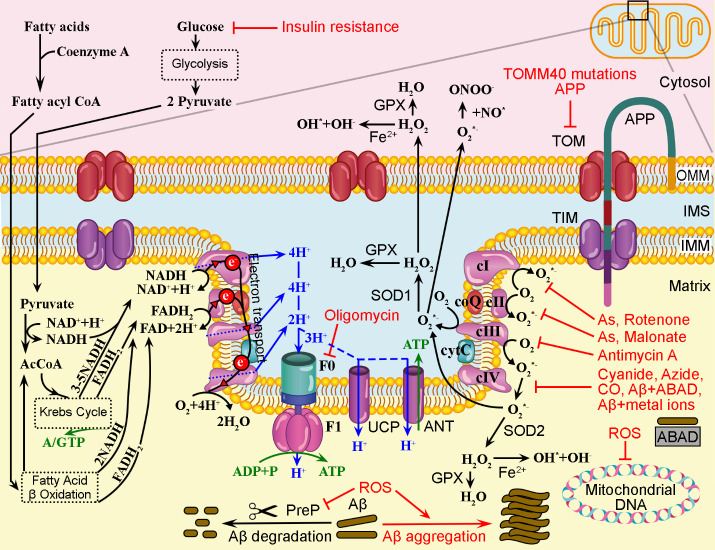
Figure 3.
Simplified schematic of the potential mitochondrial pathophysiological challenges in the CNS that may initiate AD. Represented are the main events taking place in mitochondrial cristae formed by the outer and inner mitochondrial membranes (OMM, and IMM). In physiological conditions (left), fatty acids and glucose from the cytosol fuel the Krebs cycle with Acetyl-coenzyme A (AcCoA) to generate reduced nicotinamide adenine dinucleotide (NADH) and reduced flavin adenine dinucleotide (FADH2). Oxidation of NADH provides electrons to the first membrane-bound complex (cI) in the mitochondrial respiratory chain, while oxidation of FADH2 provides electrons to cII. Electrons are transported to coenzyme Q (coQ), then to cIII, cytochrome C (cyt C), and cIV where they are used to combine oxygen (O2) and protons to form H2O. When electrons are transferred to cI, cIII, and cIV, the same complexes transport protons from the matrix into the intermembrane space (IMS) to create a proton gradient. Protons return to the matrix by passing through the ATP synthase formed of two subunits named F0 and F1, which generates ATP that can be used for biochemical reactions by cellular enzymes. Alternatively, protons can leak into the matrix via other proteins, such as uncoupling protein (UCP) and ATP exchanger adenine nucleotide translocases (ANT). Proteins produced in the cytosol can enter the IMS via translocase of the outer membrane (TOM) and the mitochondrial matrix via translocase of the inner membrane (TIM) supercomplexes. Low amounts of Aβ may also enter mitochondria, but can be degraded by Presequence protease (PreP). In unfavorable conditions (right), insulin resistance can decrease the transport of glucose into the cytoplasm of neurons, which will affect the electron transport chain and production of ATP. TOMM40 mutations and the presence of APP in the TOM/TIM supercomplexes can impede protein transport into the mitochondrial matrix. Several pollutants and toxins can inhibit the respiratory chain complexes. For example, the insecticide rotenone can inhibit the proper transport of electrons from cI, which will then combine with O2 to form superoxide radical anions (O2*-). The naturally occurring and industrial compounds malonates inhibit cII and induce the formation of O2*-. Arsenic (As) can inhibit both cI and cII. The toxin Antimycin A produced by Streptomyces bacteria can inhibit cIII. Industrial pollutants like cyanide, azide, CO, as well as endogenous Aβ bound to matrix proteins like Aβ binding alcohol dehydrogenase (ABAD, also known as 17β-hydroxysteroid dehydrogenase type 10) or metal ions can inhibit cIV. Oligomycin A, also produced by Streptomyces bacteria, is an inhibitor of F0, thus inhibits ATP production. Superoxide radical anions can be catalyzed by superoxide dismutase 1 (SOD1) in the IMS, or SOD2 in the matrix, into O2 and hydrogen peroxide (H2O2). Glutathione peroxidases (GPX) and catalases (not shown) can reduce free H2O2 to water. When ROS levels increase, anti-oxidant mechanisms become overwhelmed, which may induce the synthesis of RNS. ROS can damage DNA and RNA, as well as inhibit PreP, which will stimulate the aggregation of Aβ inside the mitochondrial matrix.