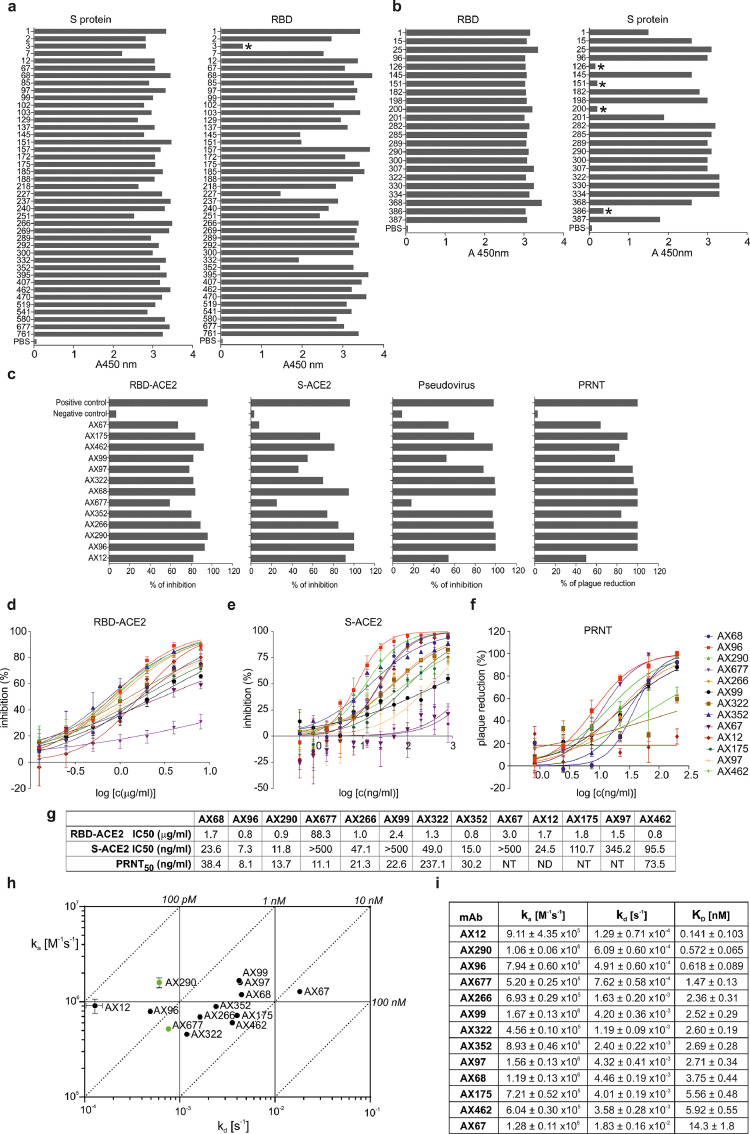
Figure 1.
Binding characteristics and inhibition activities of monoclonal antibodies specific to SARS-CoV-2 S/RBD.
(a) Immunoreactivity of hybridoma clones culture supernatants derived from mice immunized with the spike (S) protein of SARS-CoV-2 and with RBD of Spike (b) in ELISA against the pre-fusion stabilized and trimerized S protein ectodomain (“S protein”) or RBD (“RBD”). Asterisks mark non-binders.
(c) Blocking of interaction between RBD and ACE2 in a competition assay based on ELISA (RBD-ACE2), inhibition of the S protein internalization by cells expressing ACE2 (S-ACE2), inhibition of cell infection by MLV pseudotyped with SARS-CoV-2 spike protein (Pseudovirus) and plaque reduction neutralization test performed with an authentic SARS-CoV-2 virus isolate Slovakia/SK-BMC5/2020 (PRNT). Experiments were done with hybridoma culture supernatants adjusted with fresh cell culture medium to the same mAb concentration and then diluted for the assays as follows: RBD-ACE2 competitive ELISA 1:6 in PBS-T; S-ACE2 cell assay 1:50 in DMEM; pseudoviral assay 1:25 in DMEM; live virus PRNT 1:50 in EMEM. Positive control: serum of a mouse immunized with the S protein, diluted 1:200; Negative control: irrelevant mAb. All experiments are an average of at least two measurements, pseudoviral tests were all done in tetraplicates.
(d) The purified monoclonal antibodies were tested for blocking the RBD-ACE2 interaction in competitive ELISA, the inhibition of ACE2-mediated S protein internalization by HEK293/17-hACE2 cells (e), and plaque reduction neutralization test (f) performed with strain Slovakia/SK-BMC5/2020. The curves are calculated from two replicates using Prism 6 for Windows (GraphPad Software). (g) Summary of assays from d-f. (h, i) Kinetic characteristics of the interactions of RBD with selected neutralizing antibodies. On-rate (ka), off-rate (kd) constants and equilibrium dissociation constant (KD) of individual antibody-RBD complexes (±SD). Green dots mark antibodies AX290 and AX677 selected for further development.