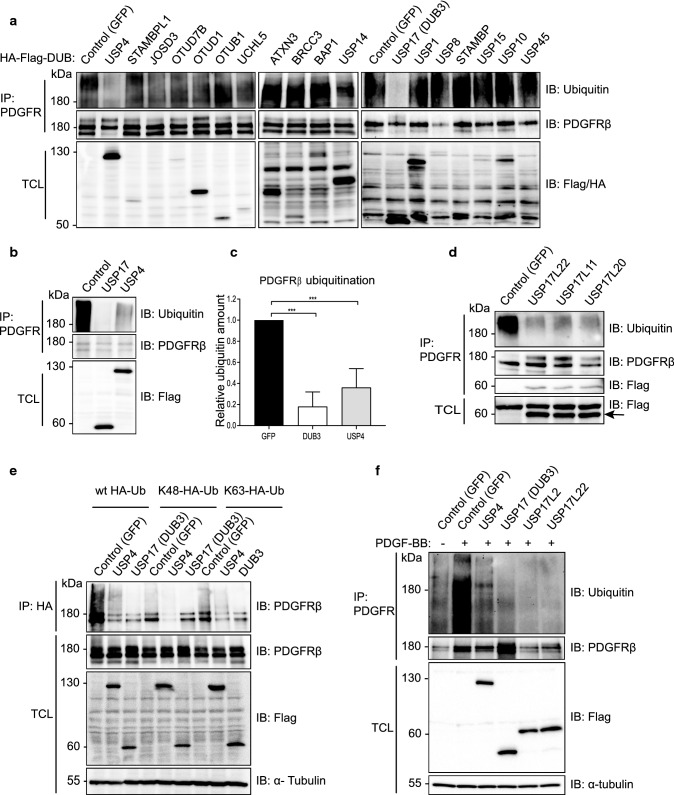
Fig. 1.
USP4 and USP17 are the deubiquitinases of PDGFRβ. a A DUB cDNA screen identifies USP17 and USP4 as deubiquitinating enzymes of PDGFRβ. Wild-type PDGFRβ and individual Flag- and HA-tagged DUB constructs were overexpressed in HEK293T, cells were serum-starved and stimulated with PDGF-BB (20 ng/ml) for 10 min, PDGFRβ was immunoprecipitated with anti-PDGFRβ antibody and eluates were immunoblotted for ubiquitin or PDGFRβ. Expression of DUBs in total cell lysates was confirmed by immunoblotting with anti-Flag (first two bottom panels) or HA (third bottom panel) antibodies. b USP4 and USP17 (DUB3) were selected and validated for the ability to remove ubiquitination from PDGFRβ. PDGFRβ was immunoprecipitated as described in panel a and eluates were immunoblotted for ubiquitin or PDGFRβ. Expression of DUBs in total cell lysates was confirmed with Flag antibody. c Quantification of PDGFRβ ubiquitination of at least three independent experiments as performed in panel b is shown; ***p < 0.001. d USP17L22, USP17L11 and USP17L20 interact with and deubiquitinate PDGFRβ. PDGFRβ and Flag-tagged USP17 isoforms were co-overexpressed in HEK293T; cells were then serum-starved and stimulated with PDGF-BB (20 ng/ml) for 10 min. PDGFRβ was immunoprecipitated and eluates were probed with a ubiquitin, PDGFRβ and Flag antibody. Expression of USP17 isoforms in total cell lysates is shown in the bottom panel. e USP4 and truncated USP17 (DUB3) remove Lys48- and Lys63-linked polyubiquitin chains from PDGFRβ. HEK293T cells were co-transfected with plasmids encoding Flag-GFP, Flag-USP4, Flag-DUB3, PDGFRβ, HA-ubiquitin or mutant HA-Lys48-Ub and HA-Lys63-Ub. Immunoprecipitation was performed with HA antibody and eluates were immunoblotted for PDGFRβ. The expression levels of PDGFRβ, Flag and α-tubulin in total cell lysates were also determined by immunoblotting. f USP4, USP17 (DUB3), full-length USP17L2 and USP17L22 isoforms remove ubiquitination from PDGFRβ under denaturing conditions. Lysates of cells that were co-expressing PDGFRβ with each of the indicated plasmids were boiled before immunoprecipitation with PDGFRβ and eluates were immunoprecipitated with HA antibodies and immunoblotted for ubiquitin and PDGFRβ. The expression levels of Flag and α-tubulin in total cell lysates were also determined by immunoblotting. IP immunoprecipitation, IB immunoblotting, TCL total cell lysates, kDa molecular mass in kilodalton