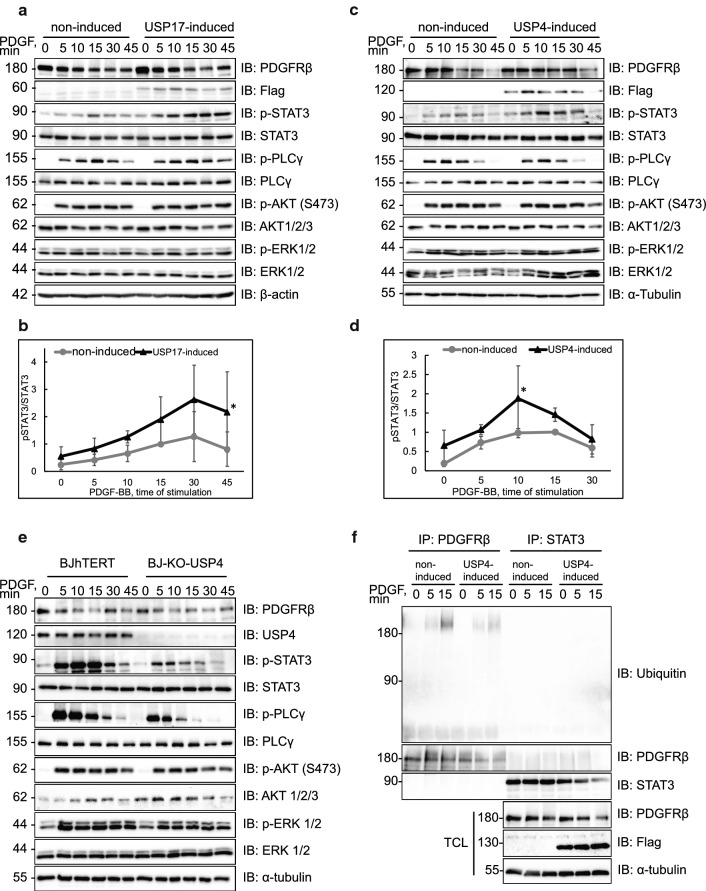
Fig. 5.
USP4 and USP17L22 affect the timing of activation of STAT3. a, b Induction of USP17L22 results in a shift of STAT3 activation to later time points in BJhTERT inducible cells. Expression of Flag-tagged USP17L22 was induced with doxycycline, cells were serum-starved and stimulated with PDGF-BB (20 ng/ml) for the indicated time periods. Expression of total and phosphorylated proteins was determined in total cell lysates using antibodies against PDGFRβ, Flag, STAT3 (pY705), STAT3, PLCγ (pY783), PLCγ, pS473 Akt1/2/3, Akt1/2/3, pThr202/pThr204 Erk1/2, Erk1/2 and β-actin (a). Phosphorylated STAT3 (pSTAT3) versus total STAT3 levels were quantified in three experiments; peak of phosphorylation at 10 min of stimulation with PDGF-BB was set as 1. Standard deviation is shown between repeats; *p < 0.05 (b). c, d Induction of USP4 increases the STAT3 activation in BJhTERT-USP4 inducible cells. BJhTERT-USP4 inducible cells were treated as described and immunoblotting was performed for proteins as in panel a; α-tubulin was used as loading control. Activated pSTAT3 relative to STAT3 protein levels were quantified in three independent experiments as in panel b; *p < 0.05 (d). e CRISPR-Cas9 knockout of USP4 decreases the STAT3 activation in BJhTERT-CRISPR-Cas9-USP4 knockout cells. The control BJhTERT and CRISPR-Cas9-USP4 knockout BJhTERT cells were starved overnight and stimulated with PDGF-BB (20 ng/ml) for the indicated time periods. Immunoblotting was performed for the indicated proteins, as in panels a–d. f USP4 does not deubiquitinate STAT3. After USP4 induction and serum starvation, BJhTERT-USP4 cells were stimulated with PDGF-BB (20 ng/ml) for the indicated time periods, lysates were divided and immunoprecipitated with anti-PDGFRβ (CTβ) or anti-STAT3 antibodies. Eluates were immunoblotted for ubiquitin. Levels of PDGFRβ, Flag-USP4 and α-tubulin were determined by immunoblotting (IB)