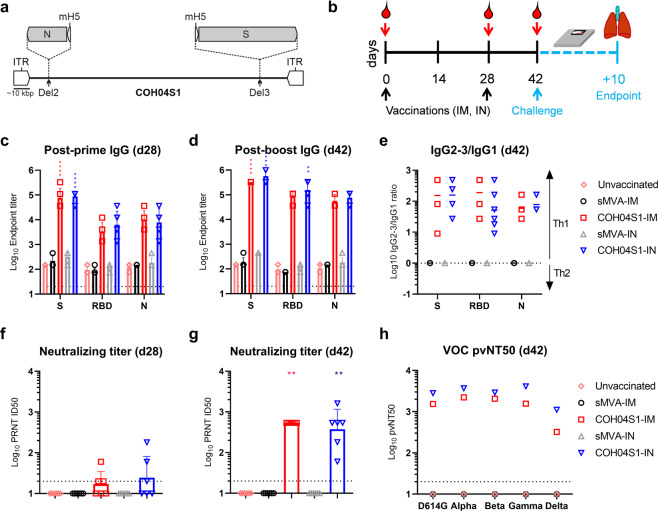
Fig. 1. COH04S1 immunogenicity in Syrian hamsters.
a COH04S1 construct. The sMVA-based COH04S1 vaccine vector co-expresses SARS-CoV-2 S and N antigen sequences that are inserted together with mH5 promoter elements into the MVA Deletion 2 (Del2) and Deletion 3 (Del3) sites as indicated. ITR inverted terminal repeat. b Study design. Hamsters (n = 6/group) were vaccinated twice with COH04S1 by IM (COH04S1-IM) or IN (COH04S1-IN) route as indicated (black arrows). Unvaccinated animals and hamsters vaccinated with empty sMVA vector by IM (sMVA-IM) or IN (sMVA-IN) route were used as controls. Blood samples were collected at day 0, 28, and 42 (red arrows). Hamsters were challenged IN at day 42 and body weight changes were recorded daily for 10 days. At endpoint, nasal wash, turbinates and lung tissue were collected for downstream analyses. c–d IgG endpoint titers. S, RBD, and N antigen-specific binding antibody titers were measured in serum samples of vaccine and control groups at day 28 (d28) post prime and at day 42 (d42) post booster immunization via ELISA. Data are presented as geometric mean values + geometric s.d. Dotted lines indicate lower limit of detection. Two-way ANOVA with Tukey’s multiple comparison test was used. e IgG2-3/IgG1 ratios. S, RBD, and N antigen-specific IgG2-3 and IgG1 endpoint titers were measured at day 42 (d42) in serum samples of vaccine and control groups and used to assess IgG2-3/IgG1 ratios. An IgG2-3/IgG1 ratio >1 is indicative of a Th1-biased response. Geometric means are indicated with a line. f–g NAb titers. NAb titers were measured in serum samples of vaccine and control groups post-first (d28) and post-second (d42) immunization via PRNT assay against SARS-CoV-2 infectious virus. Data are presented as geometric mean values + s.d. Dotted lines indicate lower limit of detection. Values below the limit of detection (ID50 = 20) are indicated as 10. One-way ANOVA with Holm-Sidak’s multiple comparison test was used. h VOC-specific NAb titers. NAb were measured in pooled serum samples of vaccine and control groups collected at the time of challenge (d42) using pseudovirus (pv) variants with S D614G mutation or S modifications based on several VOC, including Alpha (B.1.1.7), Beta (B.1.351), Gamma (P.1), and Delta (B.1.617.2). Titers are expressed as NT50. *0.05 < p < 0.01, **0.01 < p < 0.001, ***0.001 < p < 0.0001, ****p < 0.0001.