Highlights
-
•
NBTXR3 is a radioenhancer composed of functionalized hafnium oxide nanoparticles.
-
•
This report describes the first patient with pancreatic cancer treated with NBTXR3.
-
•
First demonstration of local endoscopic delivery of NBTXR3 to a deep visceral tumor.
-
•
Multidisciplinary care critical for treating pancreatic cancer patients with NBTXR3.
Abstract
Background and purpose
Pancreatic ductal adenocarcinoma (PDAC) remains one of the leading causes of cancer-related deaths in the world. For patients with PDAC who are not eligible for surgery, radiation therapy improves local disease control, yet safely delivering therapeutic doses of radiation remains challenging due to off-target toxicities in surrounding normal tissues. NBTXR3, a novel radioenhancer composed of functionalized hafnium oxide crystalline nanoparticles, has recently shown clinical activity in soft tissue sarcoma, hepatocellular carcinoma, head and neck squamous cell carcinoma, and advanced solid malignancies with lung or liver metastases. Here we report the first patient with pancreatic cancer treated with NBTXR3.
Materials and methods
A 66-year-old male with unresectable locally advanced PDAC was enrolled on our clinical trial to receive NBTXR3 activated by radiation therapy. Local endoscopic delivery of NBTXR3 was followed by intensity modulated radiation therapy (IMRT). Follow-up assessment consisted of physical examination, laboratory studies including CA19-9, and CT of the chest, abdomen, and pelvis.
Results
The patient received NBTXR3 by local endoscopic delivery without any acute adverse events. Radiation treatment consisted of 45 Gy in 15 daily fractions using IMRT. The patient began radiation twelve days after NBTXR3 injection. Daily CT-on-rails imaging demonstrated retention of NBTXR3 within the tumor for the duration of treatment. At initial follow-up evaluation, the lesion remained radiographically stable and the patient did not demonstrate treatment-related toxicity.
Conclusion
This report demonstrates initial feasibility of local endoscopic delivery of NBTXR3 activated by radiation therapy for patients with pancreatic cancer who are not eligible for surgery.
Introduction
Pancreatic ductal adenocarcinoma (PDAC) remains a major healthcare challenge, as the third leading cause of cancer-related deaths in the developed world [1]. Surgery may be curative for patients presenting with early-stage disease, but 30% of all PDAC patients are diagnosed with locally-advanced (LAPC) or borderline resectable (BRPC) disease (without distant metastatic disease) and are not eligible for upfront surgery. These patients benefit from multimodality therapy including chemotherapy and radiation therapy to improve local disease control [2], [3]. A key limitation of radiation therapy for PDAC is the potential for off-target toxicities in surrounding normal tissues. Systemic radiosensitizers make both tumors and normal tissues more radiosensitive, thus increasing toxicity. In contrast, NBTXR3, a novel radioenhancer composed of functionalized hafnium oxide crystalline nanoparticles directly injected into tumors, enhances the absorption of ionizing radiation, resulting in increased tumor cell death without adding toxicity to adjacent normal tissues [4], [5], [6]. This approach has demonstrated clinical activity in soft tissue sarcoma, and is currently being investigated across multiple tumor types [7], [8]. Here, we report the first patient treated with NBTXR3 activated by radiation therapy for PDAC, and reiterate the importance of multidisciplinary collaboration in advancing novel therapeutic avenues for this lethal disease.
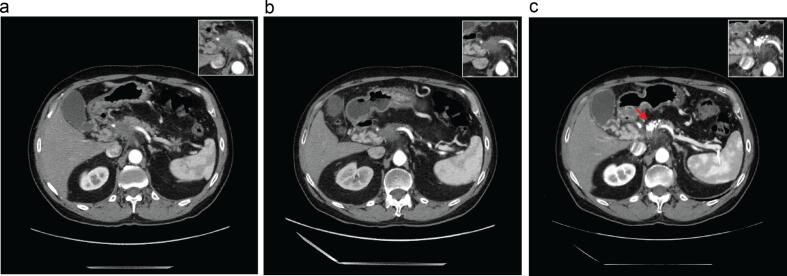
The patient is a 66-year-old male who developed epigastric discomfort, dyspepsia, and an 8-pound weight loss in the preceding 10 months. He did not have a history of cancer or smoking and consumed 2–3 drinks containing alcohol per week. He was treated for H. pylori infection by his local physician, but his symptoms persisted prompting further evaluation. Imaging studies including an MRI and CT of the abdomen and pelvis with and without contrast demonstrated a 3.1 × 2.6 cm hypodense pancreatic neck mass at the junction of the pancreatic head and pancreatic body with infiltration of the celiac trunk, origin of the splenic artery and common hepatic artery, with encasement and occlusion of the main portal vein. (Fig. 1a) He underwent an endoscopic ultrasound (EUS) and fine needle aspiration (FNA) biopsy confirming adenocarcinoma. Serum CA19-9 was 1,921 U/mL. Following review by our multidisciplinary team, the patient was staged as having unresectable LAPC and received FOLFIRINOX (5-fluorouracil, oxaliplatin, irinotecan, and leucovorin) chemotherapy. After 8 cycles of chemotherapy, CT of the chest, abdomen, and pelvis with contrast demonstrated a slight interval decrease in the size of the pancreatic mass (2.9 × 2.5 cm), and CA19-9 decreased to 170.3 U/mL. He received 3 additional cycles of chemotherapy, and imaging revealed a stable pancreatic mass without evidence of metastatic disease. In the absence of disease progression, the patient was approached to participate in our study (ClinicalTrials.gov NCT04484909) and receive NBTXR3 activated by radiation therapy.
Fig. 1.
(a) Initial staging CT of the chest, abdomen, and pelvis demonstrating an infiltrating hypodense mass in the pancreatic neck, with involvement of the celiac trunk and main portal vein. (b) Restaging CT scan after induction chemotherapy demonstrating stable disease in the pancreas. The portion of tumor shown here was determined to be a suitable area for NBTXR3 injection. (c) Restaging CT scan approximately 12 weeks after completing radiation shows a stable pancreatic mass with retained radiopaque NBTXR3. Insets in (a–c) highlight primary pancreatic tumor morphology.
At initial evaluation, the patient had recovered well from his chemotherapy and his ECOG performance status was 0. He had developed a small pulmonary embolus that was treated with enoxaparin. He reported bilateral peripheral neuropathy in his fingers and toes, and denied abdominal pain, nausea, vomiting, or shortness of breath. He had not received prior radiation therapy and had no history of gastric or duodenal ulcer disease. On physical examination, the patient was alert, had normal respiratory effort, and his abdomen was soft and non-tender to palpation. Notable laboratory values included hemoglobin 11.6 g/dL, platelets 138 K/uL, absolute neutrophil count 2.68 K/uL, creatinine 0.78 mg/dL, ALT 66 U/L, AST 56 U/L, total bilirubin 0.3 mg/dL, and CA19-9 80.8 U/mL.
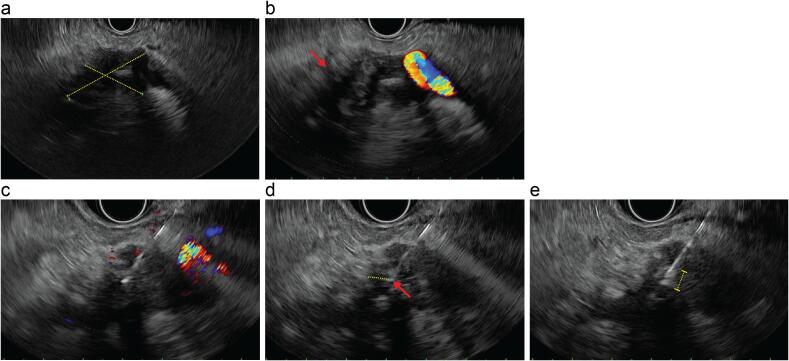
Following trial enrollment on an IRB approved protocol and informed consent, pre-treatment planning meetings with the radiation oncologist, radiologist, and gastroenterologist were conducted. We used the most recent pancreatic protocol CT scan from diagnostic radiology to estimate the GTV volume for the dose calculation of NBTXR3. The gross tumor volume (GTV) was measured to be 15.7 mL. Review of available imaging led to consensus agreement on a suitable location for NBTXR3 injection. (Fig. 1b) The patient received premedication with prednisone 30 mg at 12 h and 2 h prior to NBTXR3 administration and levofloxacin 500 mg during the procedure. EUS was performed under general anesthesia. A 3.9 × 2.8 cm hypoechoic oval mass was identified in the pancreatic neck, and FNA and core biopsies were obtained using a transgastric approach. A 22-gauge Cook needle was then inserted into the mass under color Doppler guidance to identify an avascular angle of approach, and 5.2 mL of NBTXR3 (33% of GTV) was slowly injected into the periphery and center of the tumor with three passes over >10 min, taking care to avoid blood vessels, necrotic regions, and areas <5 mm from the tumor margin. (Fig. 2; Supp. Fig. 1) Negative suction was applied on the needle for 20 s prior to withdrawal. The patient tolerated the procedure well without any acute complications or adverse events, and he was discharged home in stable condition with prescription for levofloxacin 500 mg PO for two days following the procedure.
Fig. 2.
EUS-guided intratumoral injection of NBTXR3. (a) Hypoechoic pancreatic mass measuring 3.9 cm × 2.8 cm in maximal cross-sectional diameter. Caliper dimensions shown as dashed yellow lines. (b) Mass demonstrated an irregular margin (red arrow) and encasement of the splenic artery and celiac axis. (c) An avascular plane was identified using color Doppler guidance, and needle was advanced into the tumor avoiding vascular and necrotic regions. (d) Tip of needle (red arrow) positioned at least 5 mm (dashed yellow line) from tumor margin. (e) Delivery of NBTXR3 by slow manual injection to tumor periphery and center visualized as an echogenic cloud surrounding the needle (dashed yellow line). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
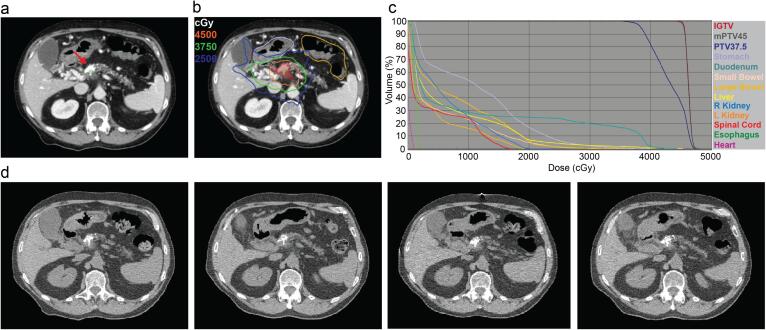
The patient underwent radiation simulation two days after NBTXR3 was injected. Respiratory-correlated breath-hold CT simulation was performed with the patient supine in a custom upper body cradle and arms positioned above his head. He was NPO for 3 h prior to simulation. Images were acquired with and without intravenous contrast, and acquisition was timed similar to a pancreatic protocol CT scan. Six breath-hold CT scans were obtained. The prescribed dose of radiation to the tumor was 45 Gy in 15 fractions, with surrounding regional lymph nodes treated to 37.5 Gy in 15 fractions using a simultaneous integrated boost technique with IMRT (6 MV photons). The CTV was defined as the GTV, celiac and SMA (from the origin of the aorta) with 1 cm margin. CT-on-rails was used for daily image guidance. All dose constraints related to target coverage (mPTV45: V45Gy > 95%; PTV37.5: V37.5 Gy > 95%) and organs at risk (Stomach: Dmax < 45 Gy; Duodenum: Dmax < 45 Gy; Small bowel: Dmax < 40 Gy; Large bowel: Dmax < 50 Gy; Heart: Dmax < 55 Gy, V40Gy < 10%, V50Gy < 1 cm3; Kidneys: V20Gy < 33%; Liver-GTV: Mean < 24 Gy; at least 700 cm3 spared below 24 Gy; Spinal cord: Dmax < 30 Gy; Spleen: Mean < 6 Gy; Chest wall: V40Gy < 150 cm3) were satisfied. (Fig. 3a-c) The proposed treatment plan was reviewed and approved at the weekly Radiation Oncology Gastrointestinal Section planning conference.
Fig. 3.
Radiation planning and delivery. (a) Radiopaque NBTXR3 visualized on simulation CT image (red arrow). (b) Representative target volumes, organs at risk, and isodose lines (IGTV: Maroon shaded; Stomach: Purple; Large bowel: Yellow-orange; 45 Gy IDL: Orange; 37.5 Gy IDL: Green; 25 Gy IDL: Blue). (c) Cumulative dose-volume histogram. (d) CT-on-rails images from (left to right) fractions 1, 5, 10, and 15 (Days 12, 16, 23, and 30 post-NBTXR3 injection) demonstrate intratumoral retention of NBTXR3 for duration of treatment course. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
The patient received his first dose of radiation twelve days after NBTXR3 injection. Radiopaque NBTXR3 was well visualized using daily CT-on-rails and was retained within the GTV for the duration of therapy with negligible intratumoral redistribution. (Fig. 3d) We did not observe NBTXR3 redistributing or accumulating in surrounding non-malignant tissues. Radiation was administered without concurrent chemotherapy. The patient was evaluated weekly during his radiation therapy. Overall, his treatment was well tolerated. He developed Grade 1 fatigue (CTCAE Version 5) and one episode of vomiting after consuming a large meal shortly after completing his sixth fraction. The skin within the radiation field showed no evidence of erythema or desquamation. He denied abdominal pain, nausea, dysphagia, diarrhea, hematemesis, hematochezia, melena, fever, or respiratory symptoms.
At his initial follow-up visit four weeks after completing radiation, the patient reported ongoing mild fatigue and peripheral neuropathy, but he continued to exercise regularly, felt his appetite had improved, and he otherwise did not demonstrate any significant radiation-related toxicities. CT of the chest, abdomen, and pelvis with and without contrast showed a stable pancreatic neck mass (2.7 × 2.6 cm) by RECIST 1.1 criteria and no evidence of metastatic disease; however, decreased enhancement and regression along the blood vessels was suggestive of an early therapeutic response. CA19-9 was 93.2 U/mL, slightly elevated compared to pre-radiation therapy levels (74.9 U/mL). Approximately twelve weeks after completing radiation, the patient continued to report mild fatigue, mild mid-abdominal pain, and peripheral neuropathy, but he did not demonstrate any treatment-related toxicities. The pancreatic lesion remained stable radiographically (Fig. 1c), and CA19-9 decreased to 59.0 U/mL.
This report describes the first patient treated with NBTXR3 activated by radiation therapy for pancreatic adenocarcinoma. Our institution is conducting a Phase I study to evaluate the safety, feasibility, and recommended Phase 2 dose for NBTXR3 in patients with LAPC or BRPC (ClinicalTrials.gov NCT04484909). We chose a hypofractionated regimen based on previously published data for locally advanced pancreatic cancer demonstrating that such regimens are safe, effective, and can be completed in a shorter time frame than conventional fractionation [9], [10]. While the regimen we used has a similar biologically effective dose to conventional fractionation (50.4–54 Gy at 1.8 Gy per fraction), we did not consider higher doses such as 67.5 Gy in 15 fractions in this study due to the known amplification of local radiation dose by NBTXR3. We believe these approaches may be feasible with SBRT, however the safety of this approach will need to be tested in a rigorous way. Planned correlative studies will evaluate biomarkers of response to treatment with NBTXR3 and radiation, including characterization of circulating tumor DNA and exosomes; quantification of immune activation (T- and B-cells, peripheral blood mononuclear cells, cytokines, T-cell receptor sequencing and repertoire); analysis of the tumor microenvironment using multiplex immunohistochemistry and hypoxia staining; and radiomics measurements of PDAC tumor morphology, including delta PDAC which measures the change in sharpness of the tumor/parenchymal interface after treatment and is associated with progression-free survival and overall survival [11], [12]. Radiation enhancement using NBTXR3 relies on a physical mechanism of activation by ionizing radiation and therefore may have broad applicability across tumor types. The feasibility of local endoscopic delivery of NBTXR3 may provide a general framework for delivering other local therapeutics including injectable toll-like receptor agonists, STING agonists, and radioactive agents such as 32P-OncoSil to deeper visceral tumors [13]. Our initial experience illustrates the critical importance of multidisciplinary evaluation and management when treating PDAC or other tumors with NBTXR3. With highly integrated care provided by radiation oncologists, gastroenterologists, radiologists, clinical research nurses and staff, and other specialists, we believe that selected patients with PDAC can safely complete EUS-guided intratumoral NBTXR3 injection and radiation therapy. Future studies are necessary to evaluate the efficacy of NBTXR3 in this setting. This report presents initial feasibility for NBTXR3 activated by radiation therapy as a potential treatment for patients with PDAC who are not eligible for surgery.
Patient consent statement
All patients involved in this research were enrolled on an institutional review board (IRB)-approved protocol and informed consent was obtained.
Funding
Trial support from Nanobiotix as part of ongoing collaborative alliance between The University of Texas MD Anderson Cancer Center and Nanobiotix (AWD0000444). The investigators were also supported by the MD Anderson Moonshot Program. Supported in part by Cancer Center Support (Core) Grant P30 CA016672 from the National Cancer Institute, National Institutes of Health, to The University of Texas MD Anderson Cancer Center.
Declaration of Competing Interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: A.M. reports royalties for license from Cosmos Wisdom Biotechnology Ltd. and license for patent from Thrive Earlier Detection, all outside the submitted work. B.D.M. has served as the Co-chair of the Gastrointestinal Steering Committee at the National Cancer Institute (NCI) and has received grants from National Institutes of Health (NIH) and NCI (NIH/NCI 2U19CA021239-35, 1U10CA180858-01), all outside the submitted work. G.L.S. has received grants from NCI, Radiation Oncology Institute, and MD Anderson internal funding, all outside the submitted work. P.D. reports consulting fees from Leidos Biomedical Research, payment for educational events from ASCO, ASTRO, Conveners, and Physicians Education Resource, and participation on an advisory board for Adlai Nortye, all outside the submitted work. A.C.K. reports stock from Aravive, Inc., outside the submitted work. C.M.T. serves on the clinical advisory board for Accuray, Inc., outside the submitted work. E.P.T. reports in kind research funding from General Electric, outside the submitted work. M.S.B. reports institutional research support from Nanobiotix and from Oncosil, Galera, Augmenix, Silenseed, outside the submitted work. E.J.K. reports funding support for the trial from Nanobiotix, and sponsored research funding from Philips Healthcare and GE Healthcare, grant funding from Stand Up 2 Cancer, royalties for book from Taylor and Francis LLC, consulting feeds from RenovoRx, pending patent for 3D-printed oral stents, and a leadership role in International Cholangiocarcinoma Research Network as radiation oncology representative, outside the submitted work. The authors otherwise have no disclosures to report.
Acknowledgements
The authors acknowledge Maria Jovie Rodriguez, Ryan T. Mathew, Cassidy Papso, and Dr. Mark Hurd for their critical roles in the support of this study. The authors also acknowledge the Cancer Moonshot program at The University of Texas MD Anderson Cancer Center.
Contributor Information
Manoop S. Bhutani, Email: manoop.bhutani@mdanderson.org.
Eugene J. Koay, Email: ekoay@mdanderson.org.
References
- 1.SEER Cancer Stat Facts: Pancreatic Cancer. National Cancer Institute (2020). Available at: https://seer.cancer.gov/statfacts/html/pancreas.html. (Accessed: 18th January 2021).
- 2.Hammel P., et al. Effect of chemoradiotherapy vs chemotherapy on survival in patients with locally advanced pancreatic cancer controlled after 4 months of gemcitabine with or without erlotinib the LAP07 randomized clinical trial. JAMA - J Am Med Assoc. 2016;315:1844–1853. doi: 10.1001/jama.2016.4324. [DOI] [PubMed] [Google Scholar]
- 3.Katz M.H.G., et al. Borderline Resectable Pancreatic Cancer: The Importance of This Emerging Stage of Disease. J Am Coll Surg. 2008;206:833–846. doi: 10.1016/j.jamcollsurg.2007.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maggiorella L., et al. Nanoscale radiotherapy with hafnium oxide nanoparticles. Futur Oncol. 2012;8:1167–1181. doi: 10.2217/fon.12.96. [DOI] [PubMed] [Google Scholar]
- 5.Pottier A, Borghi E, Levy L. New use of metals as nanosized radioenhancers. In: Anticancer Research 34, 443–453, International Institute of Anticancer Research; 2014. [PubMed]
- 6.Schuemann J., Bagley A.F., Berbeco R., Bromma K., Butterworth K.T., Byrne H.L., et al. Roadmap for metal nanoparticles in radiation therapy: current status, translational challenges, and future directions. Phys Med Biol. 2020;65(21):21RM02. doi: 10.1088/1361-6560/ab9159. [DOI] [PubMed] [Google Scholar]
- 7.Bonvalot S., Rutkowski P.L., Thariat J., Carrère S., Ducassou A., Sunyach M.-P., et al. NBTXR3, a first-in-class radioenhancer hafnium oxide nanoparticle, plus radiotherapy versus radiotherapy alone in patients with locally advanced soft-tissue sarcoma (Act. In.Sarc): a multicentre, phase 2–3, randomised, controlled trial. Lancet Oncol. 2019;20(8):1148–1159. doi: 10.1016/S1470-2045(19)30326-2. [DOI] [PubMed] [Google Scholar]
- 8.Bonvalot S., et al. First-in-human study testing a new radioenhancer using nanoparticles (NBTXR3) activated by radiation therapy in patients with locally advanced soft tissue sarcomas. Clin Cancer Res. 2017;23:908–917. doi: 10.1158/1078-0432.CCR-16-1297. [DOI] [PubMed] [Google Scholar]
- 9.Reyngold M., et al. Association of ablative radiation therapy with survival among patients with inoperable pancreatic cancer. JAMA Oncol. 2021;7:735–738. doi: 10.1001/jamaoncol.2021.0057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Krishnan S., et al. Focal radiation therapy dose escalation improves overall survival in locally advanced pancreatic cancer patients receiving induction chemotherapy and consolidative chemoradiation. Int J Radiat Oncol Biol Phys. 2016;94:755–765. doi: 10.1016/j.ijrobp.2015.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Koay E.J., et al. A visually apparent and quantifiable CT imaging feature identifies biophysical subtypes of pancreatic ductal adenocarcinoma. Clin Cancer Res. 2018;24:5883–5894. doi: 10.1158/1078-0432.CCR-17-3668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Amer A.M., Zaid M., Chaudhury B., Elganainy D., Lee Y., Wilke C.T., et al. Imaging-based biomarkers: Changes in the tumor interface of pancreatic ductal adenocarcinoma on computed tomography scans indicate response to cytotoxic therapy. Cancer. 2018;124(8):1701–1709. doi: 10.1002/cncr.31251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Naidu J., et al. Combined chemotherapy and EUS-guided intra-tumoral 32-P implantation for locally advanced pancreatic ductal adenocarcinoma: a pilot study. Endoscopy. 2021 doi: 10.1055/a-1353-0941. [DOI] [PubMed] [Google Scholar]