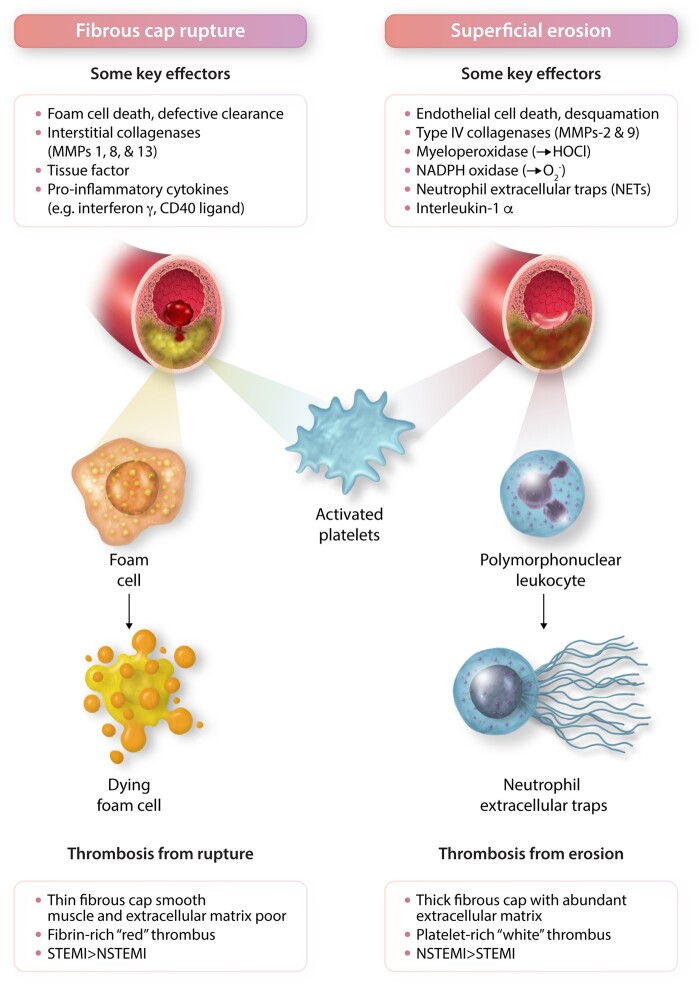
Figure 2.
Contrasting mechanisms of plaque disruption due to fibrous cap rupture vs. superficial erosion. These two forms of plaque disruption involve distinct vascular cellular protagonists: Smooth muscle cells produce the interstitial collagens that lend strength to the plaque’s fibrous cap. Plaque regions depleted of smooth muscle cells have impaired ability to repair and maintain the collagenous extracellular matrix of the plaque’s protective fibrous cap. In contrast, endothelial cell death and desquamation proves pivotal in plaque disruption due to superficial erosion. The interstitial collagenases, matrix metalloproteinases (MMPs) -1, 8, and -13, attack the fibrillar collagen (types I and III) mostly produced by smooth muscle cells that protect the plaque from rupture. In contrast, the Type IV collagenases, MMPs -2 and -9, degrade the non-fibrillar Type IV collagen in the basement membrane underlying the endothelial cell monolayer thus dissolving the substrate on which endothelial cells attach to the intimal surface. Deprived of their extracellular matrix substrate, endothelial cells can undergo death by anoikis and slough more readily. Oxidative stress due to hypochlorous acid (HOCl) generated by myeloperoxidase or superoxide anion ( produced by NADPH oxidase can damage the endothelial monolayer and promote desquamation. Pro-inflammatory cytokines including interleukin-1β, tumour necrosis factor, and CD40 ligand participate in plaque rupture by inducing the interstitial collagenases and tissue factor, the instigator of thrombosis in ruptured plaques. In superficial erosion, NETs contribute to thrombosis and can amplify and propagate endothelial damage through NET-associated interleukin-1α. The main cellular effectors of rupture vs. erosion differ as well, foam cells derived from monocytes or smooth muscle cells predominate in the pathophysiology of fibrous cap rupture. These cells can eventually die from apoptosis or oncosis. In superficial erosion, polymorphonuclear leucocytes appear prominent and can undergo NET formation. Activated platelets contribute to thrombus formation and growth in both forms of plaque disruption. However, thrombi produced by plaque rupture appear more fibrin rich, entrapping erythrocytes, forming ‘red’ thrombi. In contrast, plaques disrupted by erosion tend to generate platelet-rich ‘white’ thrombi. As noted in Figure 1, fibrous cap rupture causes ST segment elevation (STEMI) more commonly than non-ST segment myocardial infarction (NSTEMI) whereas eroded lesions more frequently associate with NSTEMI than STEMI.