Rheumatology key message
Ticagrelor added to methotrexate may improve rheumatoid arthritis disease severity.
Dear Editor, Low-dose weekly MTX remains the anchor drug for treatment of RA. One mechanism by which MTX suppresses inflammation in RA is enhancing systemic adenosine release, which suppresses inflammation by stimulating adenosine receptors on T cells, macrophages and other inflammatory cells. Many RA patients exhibit a poor response to low-dose MTX, and murine findings suggest that MTX resistance may be due to inadequately increased adenosine release [1]. Because adenosine is primarily taken up from the extracellular space via the nucleoside transporter equilibrative nucleoside transporter 1 (ENT1), we asked whether an agent that blocks adenosine uptake enhances the effect of MTX in the treatment of RA [2]. We therefore conducted a 1-month proof-of-concept clinical trial to investigate whether ticagrelor (an inhibitor of the adenosine transporter ENT1) and platelet P2Y12 inhibitor [used to prevent cardiovascular (CV) events] improves RA severity in those on low-dose MTX [2].
Ticagrelor in Methotrexate-Resistant Rheumatoid Arthritis—NCT02874092 enrolled RA patients without clinical CV disease if they met ACR criteria for RA, and had active disease defined by a DAS28-ESR > 3.6 on stable doses of MTX monotherapy for a minimum of 12 weeks. RA participants entered a 1-month open-label protocol in which they were administered ticagrelor (90 mg) twice daily. Disease activity, including DAS28-ESR, and the number of tender and swollen joints, were assessed by a board-certified rheumatologist. This study was approved by the NYU Langone Health–Bellevue institutional review board, and all subjects provided written informed consent before participation, consistent with the Declaration of Helsinki.
Platelet activation, indicated by P-selectin and PAC-1 and aggregation to adenosine diphosphate (ADP), was performed as previously described (Supplementary Data S1, available at Rheumatology online) [3]. ESR values were obtained from the clinical laboratory. To measure plasma adenosine, HPLC with concentration (nM) determined by comparison to standards was performed (Supplementary Data S1, available at Rheumatology online) [4].
Ticagrelor in Methotrexate‐Resistant Rheumatoid Arthritis was initially powered to detect at least a 50% reduction in ADP‐induced platelet aggregation and a >1.2 unit improvement in DAS28 score after 1‐month of ticagrelor added to MTX therapy. A recruitment goal of n = 23 subjects was intended to provide 80% power to detect these differences at a 0.05 significance level. The study was stopped after 1 year due to slow enrolment (full details in Supplementary Data S1, available at Rheumatology online).
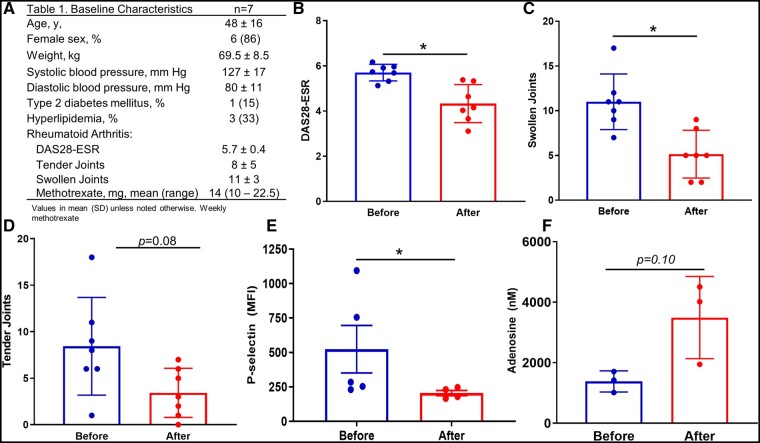
The baseline characteristics of the study participants are listed in Fig. 1A. A total of 7 RA patients were enrolled who were young [mean age 48 (16) years], predominantly female (86%) and who had a low prevalence of traditional CV risk factors and high RA disease activity. After 1 month of ticagrelor, 6 (of 7) RA patients displayed improvement in the DAS28-ESR score, including 4 achieving a reduction of >1.2 and 2 patients achieving a reduction of >0.6 but <1.2 response (Fig. 1B). Individually, a reduction in swollen (11 → 5, P = 0.02, Fig. 1C) and borderline reduction in tender (8 → 3, P = 0.08, Fig. 1D) joints was also observed.
Fig. 1.
Ticagrelor added to low-dose MTX improves RA severity and reduces platelet activation
(A) Baseline characteristics of RA patients at study enrolment. Change in RA severity as assessed by (B) DAS28-ESR, (C) number of swollen and (D) tender joints at baseline (before) and after 1 month of 90 mg twice daily ticagrelor. (E) Platelet expression by flow cytometry of P-selectin. (F) Serum adenosine measured through HPLC. MFI; mean fluorescent intensity. P-value indicates Wilcoxon paired signed-rank test (P-selectin—Mann-Whitney U test). N = between 4 and 7 measures/group. *P < 0.05.
As expected, light transmission aggregometry to both low (2 µM) and high (5 µM) concentrations of ADP revealed significant platelet inhibition after ticagrelor therapy, demonstrating study drug compliance (Supplementary Fig. S1, available at Rheumatology online). Furthermore, other markers of platelet activation, including P-selectin (Fig. 1E) and PAC-1 after ADP stimulation (Supplementary Fig. S1C/D, available at Rheumatology online), were reduced. Finally, in a subset of 3 participants who underwent adenosine measurement, adenosine trended upwards (Fig. 1F) following 1 month of ticagrelor.
MTX remains the first-line therapy for the treatment of RA, yet despite this, many RA patients will require further therapies to achieve appropriate disease control [5]. MTX indirectly inhibits AMP deaminase and adenosine deaminase, thereby leading to increased accumulation of intracellular adenosine, with subsequent extracellular adenosine release [6]. In fibroblast-like synoviocytes and inflammatory cells, MTX-driven adenosine release activates inflammatory cell adenosine receptors, leading to inhibition of NFkB-driven processes [6]. In murine models, diminished MTX-induced adenosine accumulation was shown to be one mechanism leading to resistance to the anti-inflammatory properties of MTX [4]. Ticagrelor is a potent P2Y12 receptor blocker that inhibits platelet aggregation and is commonly used in CV disease prevention, and it also increases extracellular adenosine, thus providing mechanistic rationale for our study [2].
Limitations of our study include its observational nature (with lack of a control group), small sample size (thus potentially allowing a type I error), lack of blinding (which could lead to observation bias) and that other medications exist and are approved for RA treatment that do not enhance bleeding risk. However, RA is a known CV risk factor. Reducing RA disease activity is linked to a reduction in CV events [7], and many RA patients may require anti-platelet therapy for other clinical indications to prevent CV events, thus potentially supporting a unique indication for ticagrelor. In conclusion, in this pilot open-label clinical trial, 1 month of ticagrelor therapy added to weekly MTX significantly reduced RA disease severity, platelet aggregation and platelet activation, while showing a trend towards increase in circulating adenosine. Larger studies are needed to assess the benefit vs the risk of such an approach.
Supplementary Material
Acknowledgements
J.S.B. was supported, in part, by National Institutes of Health (Bethesda, MD) grants R01HL139909 and R35HL144993. B.N.C. was supported, in part, by grants from the National Institute of Arthritis, Musculoskeletal and Skin Diseases (RO1-AR054897 and RO1-AR056672), and the NYU-HHC Clinical and Translational Science Institute (UL1-TR000038 from the National Center for Advancing Translational Sciences).
Funding: This study was supported by an investigator-initiated grant provided by AstraZeneca to J.S.B. and B.N.C. Other support, in part, included an American Heart Association Career Development Grant (Dallas, TX) (18CDA34080540) and a National Institutes of Health (Bethesda, MD) K23 Career Development Award (HL152013-01A1), each awarded to M.S.G.
Disclosure statement: J.S.B is on the advisory boards of Janssen Pharmaceutical and Amgen. B.N.C. is an inventor on a number of patents and owns equity in Regenosine, a company developing therapy for OA. The other authors have declared no conflicts of interest.
Data availability statement
The additional data that support the findings of this study are available from the corresponding author upon reasonable request.
Trial registration: ClinicalTrials.gov, https://clinicaltrials.gov, NCT02874092.
Contributor Information
Michael S Garshick, Center for the Prevention of Cardiovascular Disease, Department of Medicine; Leon H. Charney Division of Cardiology, Department of Medicine.
Pamela B Rosenthal, Division of Rheumatology, Department of Medicine.
Elliot Luttrell-Williams, Leon H. Charney Division of Cardiology, Department of Medicine.
Bruce N Cronstein, Division of Rheumatology, Department of Medicine; Division of Translational Medicine.
Jeffrey S Berger, Center for the Prevention of Cardiovascular Disease, Department of Medicine; Leon H. Charney Division of Cardiology, Department of Medicine; Division of Vascular Surgery, Department of Surgery, New York University School of Medicine, New York, NY, USA.
Supplementary data
Supplementary data are available at Rheumatology online.
References
- 1. You X, Williams A, Dervieux T, He W, Cronstein BN. Fibroblasts from methotrexate-sensitive mice accumulate methotrexate polyglutamates but those from methotrexate-resistant mice do not. Clin Exp Rheumatol 2013;31:433–5. [PMC free article] [PubMed] [Google Scholar]
- 2. Cattaneo M, Schulz R, Nylander S. Adenosine-mediated effects of ticagrelor: evidence and potential clinical relevance. J Am Coll Cardiol 2014;63:2503–9. [DOI] [PubMed] [Google Scholar]
- 3. Nhek S, Clancy R, Lee KA et al. Activated platelets induce endothelial cell activation via an interleukin-1beta pathway in systemic lupus erythematosus. Arterioscler Thromb Vasc Biol 2017;37:707–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Corciulo C, Lendhey M, Wilder T et al. Endogenous adenosine maintains cartilage homeostasis and exogenous adenosine inhibits osteoarthritis progression. Nat Commun 2017;8:15019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Singh JA, Saag KG, Bridges SL Jr et al. 2015 American College of Rheumatology guideline for the treatment of rheumatoid arthritis. Arthritis Rheumatol 2016;68:1–26. [DOI] [PubMed] [Google Scholar]
- 6. Cronstein BN, Aune TM. Methotrexate and its mechanisms of action in inflammatory arthritis. Nat Rev Rheumatol 2020;16:145–54. [DOI] [PubMed] [Google Scholar]
- 7. Solomon DH, Reed GW, Kremer JM et al. Disease activity in rheumatoid arthritis and the risk of cardiovascular events. Arthritis Rheumatol 2015;67:1449–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The additional data that support the findings of this study are available from the corresponding author upon reasonable request.
Trial registration: ClinicalTrials.gov, https://clinicaltrials.gov, NCT02874092.