Abstract
Background
The coronavirus 2019 (COVID-19) pandemic upset healthcare systems and their logistics worldwide. We sought to assess safety and effectiveness of an optimized logistics for transcatheter aortic valve implantation (TAVI) pathway developed during the COVID-19 pandemic.
Methods
This is a retrospective analysis. An optimized TAVI logistics based on performing TAVI work-up and procedure during the same hospitalization was used during the COVID-19 pandemic. In-hospital and 30-day outcomes of patients treated during the pandemic were compared with an historical cohort of patients undergoing TAVI with staged work-up before the pandemic within an homogeneous timeframe.
Results
Of 536 patients, 227 (42.4%) underwent TAVI during the COVID-19 pandemic with a reduction of 26.5% compared to the pre-pandemic period (n = 309). The median age was 81 (77–85) years and STS score was 3.4 (2.2–5.6)%. Lower rates of in-hospital major vascular complications (2.2% vs. 8.7%; p < 0.01) and life-threatening bleeding (0.4% vs. 4.2%; p = 0.01) were reported in the COVID-19 period, whereas no difference in acute kidney injury (7.0% vs. 7.4%, p = 0.85) rate was reported between COVID-19 and pre-COVID-19 periods. No difference in 30-day rates of all-cause death (4.0 vs. 4.5, p = 0.75) and of major adverse cardiovascular events (4.0 vs. 6.1, p = 0.26) were reported between COVID-19 and pre-COVID-19 periods.
Conclusions
The use of optimized single-hospitalization logistics for TAVI workup and procedure developed during the COVID-19 pandemic, showed to be as safe and effective as the two-stage TAVI pathway previously adopted, allowing the minimization of potential exposure to COVID-19 infection and shortening times to treatment for severely symptomatic patients.
Keywords: TAVI, COVID-19, Logistics, Optimization, Outcomes
Graphical abstract
1. Introduction
The coronavirus disease 2019 (COVID-19) has caused a global pandemic, resulting in millions of deaths and in a dramatic burden on healthcare [1]. Alongside with the COVID-related deaths, the fear to be in touch with COVID-19 carriers and therefore to be infected, has led a large portion of patients affected by serious diseases to deny or post-pone access to emergency departments, thus resulting in an increased side-mortality for many patients, in particular for those affected by cardiovascular diseases [2,3].
Patients with severe aortic stenosis (AS) have a poor prognosis if left untreated. Despite transcatheter aortic valve implantation (TAVI) offers a less invasive treatment with a fast recovery for these patients [4], imposed health-care restrictions related to COVID-19 pandemic needs let a not-negligible portion of TAVI candidates to be deferred during pandemic waves, worsening the outcomes of these patients [5].
In our tertiary care hospital, we remodelled the logistics of TAVI program during COVID-19 pandemic to accomplish technical and administrative restrictions imposed by national and regional emergency laws, and to maintain offering a safe and timely treatment for patients with a minimal use of hospital resources. The purpose of this study was to demonstrate the feasibility, safety and effectiveness of the optimized TAVI logistics adopted during the COVID-19 pandemic.
2. Methods
2.1. Data source
We retrospectively analyzed outcomes of patients undergoing TAVI from March 15th 2020 to April 2021 (COVID-19 period group) and compared with those of patients undergoing TAVI in the preceding same-length period from February 2019 to March 14th 2020 (pre-COVID period group), in our institute (Policlinico G. Rodolico-San Marco Hospital, Catania). The used cut-off date matches with the day on which the Italian government declared the first national lockdown in Italy for COVID-19 pandemic, and consequently with the beginning of social, work and health-care restrictions.
All consecutive patients undergoing TAVI were included in the present analysis. All patients without severe renal impairment (chronic kidney disease stage IV or V), underwent preprocedural assessment with ECG-gated computed tomographic angiography (CTA) as per standard practice. All commercially available devices for TAVI were used according instruction for uses and operator experience. Outcomes and complications were assessed according Valve Academic Research Consortium-2 (VARC-2) definitions [6]. Follow-up was performed at thirty days by in person visits (during pre-pandemic period) or telephonic follow up (during the pandemic).
All subjects provided written informed consent for the procedure. The study was conducted according to the principles of the Declaration of Helsinki and Good Clinical Practice. The study did not undergo ethical committee approval, considering the retrospective nature of the analysis.
2.2. Logistics during COVID-19 period
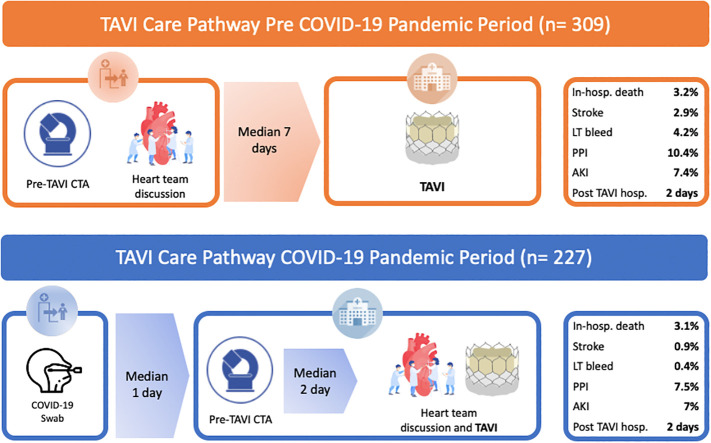
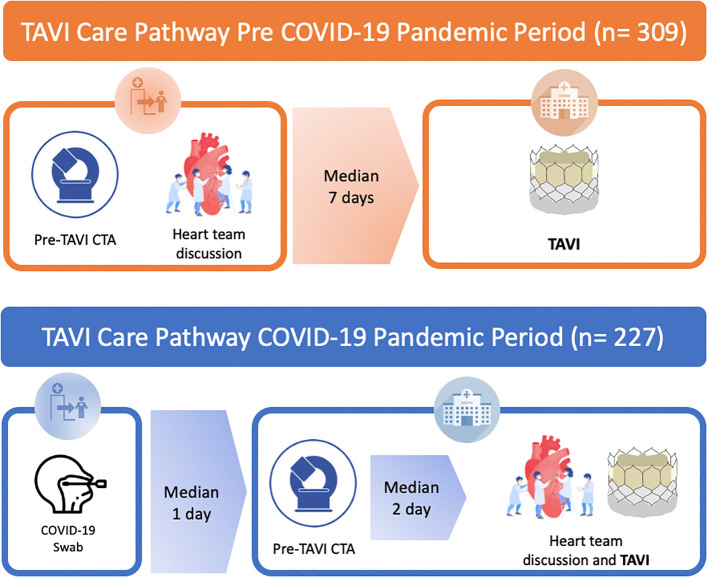
COVID-19 pandemic-related changes in TAVI logistics were forced since March 15th 2020. Several adjustments to the TAVI work-up aimed at minimizing hospital stay, reducing potential staff and patient exposure to COVID-19 infection were initiated since the aforementioned date (Fig. 1 ).
Fig. 1.
TAVI care pathway with changes made during the COVID-19 pandemic.
The illustration shows the same TAVI care pathway but first one (orange) is before and the second one is after the COVID-19 pandemic (blue). Looking first at the one before the COVID pandemic (orange), we can see that the staged pre-procedural planning for TAVI (CTA and discussion by local Heart Team) was done in a different hospitalization (as an outpatient). TAVI procedure was performed in another hospitalization. Moving on to the second picture (blue), we can see that the day before hospitalization, patients were tested for SARS-CoV-2 by real-time reverse transcription polymerase chain reaction (rRT-PCR) with a swab. Pre-procedural CTA was performed during the same hospitalization, on the admission day. Case discussion by Heart-Team was carried on the same morning of TAVI procedure. TAVI was performed two days (median) after the admission day. Abbreviations: TAVI, Transcatheter Aortic Valve Implantation; COVID, CoronaVirus Disease-19; CTA, Computed Tomography Angiography; LT, Life-Threatening; PPI, Permanent Pacemaker Implantation; AKI, acute kidney injury. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
The following changes in TAVI practice were introduced:
-
•
The day before hospitalization, patients were screened for symptoms according to CDC guidelines, tested for SARS-CoV-2 by real-time reverse transcription polymerase chain reaction (rRT-PCR) and underwent routine blood sampling.
-
•
Pre-procedural CTA was systematically performed during the same hospitalization, on the admission day.
-
•
Feasibility of TAVI procedure was assessed, and case discussion by Heart-Team was carried on the same day of TAVI procedure, that used to be the working day after the admission.
-
•
WHO's recommendations for the rational use of personal protective equipment (PPE) in health care (patients and operators) were applied during the in-hospital stay.
2.3. Statistical analysis
Continuous variables were presented as median and interquartile range (IQR), and compared using the Mann-Whitney U test.
Categorical variables were presented as frequencies and percentages and compared using the Pearson's chi-square test. All statistical tests were performed two-tailed, and a p-value <0.05 was considered as the threshold for statistical significance. Statistical analyses were performed using IBM SPSS Statistics 25.0 (IBM Corporation, New York, USA).
3. Results
3.1. Baseline, pre-procedural and procedural characteristics
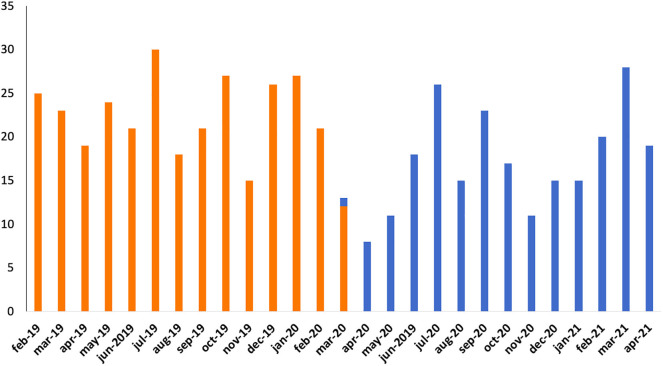
A total of 536 patients were included in the present analysis. Among these, 227 (42.4%) underwent TAVI during the COVID 19 pandemic between March 15th 2020 and April 2021, whereas 309 (57.7%) underwent TAVI before the pandemic during an equivalent time period, from February 2019 to March 14th 2020. A 26.5% reduction of TAVI procedures was then observed (Supplementary Fig. 1).
Supplementary Fig. 1.
Number of TAVI per month during the study period: pre-COVID period (orange) and COVID period (blue). Abbreviations: TAVI, Transcatheter Aortic Valve Implantation; COVID, CoronaVirus Disease-19.
Patients had a median age and a STS Mortality score of 81 (77–85) years and 3.3 (2.2–4.8)%, respectively, and were in New York Heart Association (NYHA) class III/IV before TAVI in most of cases (n = 365, 68.1%). At baseline, patients treated during the COVID-19 period had a higher incidence of prior myocardial infarction (MI) (17.2% vs. 9.4%, p < 0.01). Baseline demographic, clinical and echocardiographic characteristics are summarized in Table 1 .
Table 1.
Baseline characteristics of pre-COVID and COVID period patients.
| All patients (n = 536) | Pre-COVID period (n = 309) | COVID period (n = 227) | p-Value | |
|---|---|---|---|---|
| Age (years), median (IQR) | 81 (77–85) | 82 (78–85) | 81 (77–85) | 0.28 |
| Female, n (%) | 316 (59.0) | 182 (58.9) | 134 (59.0) | 0.98 |
| STS Mortality Score, median (IQR) | 3.3 (2.2–4.8) | 3.3 (2.3–4.9) | 3.1 (2.0–4.7) | 0.24 |
| BMI, median (IQR) | 27 (24–30) | 27 (24–30) | 27 (23−30) | 0.71 |
| Hypertension, n (%) | 468 (87.3) | 272 (88.0) | 196 (86.3) | 0.56 |
| Diabetes, n (%) | 195 (36.4) | 110 (35.6) | 85 (37.4) | 0.66 |
| Prior MI, n (%) | 68 (12.7) | 29 (9.4) | 39 (17.2) | <0.01 |
| Prior CABG, n (%) | 28 (5.2) | 14 (4.5) | 14 (6.2) | 0.40 |
| Prior PCI, n (%) | 84 (15.7) | 44 (14.2) | 40 (17.6) | 0.29 |
| Prior stroke, n (%) | 24 (4.5) | 14 (4.5) | 10 (4.4) | 0.95 |
| Prior pacemaker, n (%) | 40 (7.5) | 25 (8.1) | 15 (6.6) | 0.52 |
| COPD, n (%) | 79 (14.7) | 50 (16.2) | 29 (12.8) | 0.27 |
| AF, n (%) | 132 (24.6) | 78 (25.2) | 54 (23.8) | 0.70 |
| NYHA class ≥ III, n (%) | 365 (68.1) | 218 (70.6) | 147 (64.8) | 0.14 |
| Renal failure, n (%) | 75 (14.0) | 39 (12.6) | 36 (15.9) | 0.29 |
| Anticoagulant, n (%) | 113 (21.1) | 62 (20.1) | 51 (22.5) | 0.50 |
| SAPT, n (%) | 246 (45.9) | 122 (39.5) | 124 (54.6) | 0.01 |
| DAPT, n (%) | 37 (6.9) | 9 (2.9) | 28 (12.3) | <0.01 |
| DAT, n (%) | 17 (3.2) | 5 (1.6) | 12 (5.3) | 0.02 |
| Echocardiographic parameters | ||||
| LVEF, median (IQR) | 55 (48–60) | 55 (50–60) | 55 (48–60) | 0.66 |
| Mean Gradient, mmHg, median (IQR) | 46 (39–56) | 47 (38–55) | 46 (39.5–56.5) | 0.98 |
| AVA, cm2, median (IQR) | 0.6 (0.5–0.7) | 0.6 (0.5–0.7) | 0.6 (0.5–0.7) | 0.14 |
Abbreviations: AF, Atrial Fibrillation; AVA, Aortic Valve Area; BMI, Body Mass Index; CABG, Coronary Artery Bypass Graft; COPD; Chronic Obstructive Pulmonary Disease; IQR, InterQuartile Range; DAPT, Dual Antiplatelet Therapy; DAT, Dual Therapy; LVEF, Left Ventricle Ejection Fraction; MI, Myocardial Infarction; NYHA, New York Heart Association; PCI, Percutaneous Coronary Intervention; SAPT, Single Antiplatelet Therapy; STS, Society of Thoracic Surgery.
Time from diagnosis of severe aortic stenosis to TAVI hospitalization was shorter in the COVID-19 period [40 (36.5–58) vs. 19 (10.75–29.25), p < 0.01].
During the COVID-19 pandemic, patients underwent pre-procedural CTA assessment in a higher percentage of cases compared to pre-pandemic period (97.4% vs. 92.6%, p < 0.01), with the vast majority undergoing CTA during the same hospitalization (81.9% vs. 45.6%, p < 0.01). Times between pre-procedural CTA and TAVI [2 (1–4) vs. 7 (2–28), p < 0.01] was shorter during the pandemic. Pre-procedural CTA characteristics are shown in Supplementary Table 1. No patients admitted during the COVID-19 period were found ineligible for TAVI.
The transfemoral approach was the most used TAVI access during both periods taken into account (n = 532, 99.3%). Procedural characteristics are reported in Supplementary Table 2.
3.2. Postprocedural outcomes
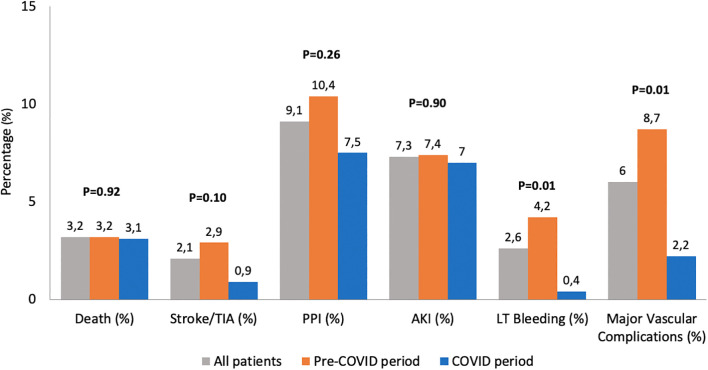
Postprocedural outcomes are presented in Fig. 2 and Supplementary Table 3. High rates of device success were reported in both time periods taken into account (96.5% vs. 95.8% for pandemic and pre-pandemic periods respectively, p = 0.69). During hospitalization, no differences in all-cause death (3.1 vs. 3.2, p = 0.92), cardiovascular death (2.6 vs. 2.6, p = 0.97), stroke (0.9 vs. 2.9, p = 0.10), MI (0.0 vs. 0.6, p = 0.23), permanent pacemaker implantation (PPI) (7.5 vs. 10.4, p = 0.26), and acute kidney injury (AKI) (7.0% vs. 7.4%, p = 0.85) were reported between pandemic and pre-pandemic periods. Lower rates of life-threatening bleeding (0.4% vs. 4.2%, p = 0.01), major vascular complication (2.2% vs. 8.7%, p < 0.01) and left bundle branch block (LBBB) (20.7% vs. 28.5%, p = 0.04) were reported in COVID-19 period patients.
Fig. 2.
Procedural Outcomes during COVID (blue) and pre-COVID period (orange). Abbreviations: COVID, CoronaVirus Disease-19; TIA Transient Ischemic Attack; AKI, acute kidney injury; PPI, Permanent Pacemaker Implantation; LT, Life-Threatening. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
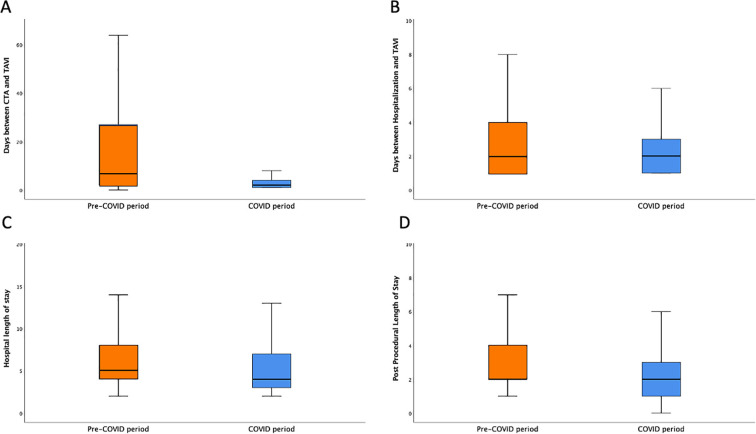
During COVID-19 pandemic, shorter postprocedural and total length of stay [2 (1–3) vs. 2 (2–4) days, and 4 (3–7) vs. 5 (4–8) days respectively, p < 0.01 for both] were reported (Supplementary Fig. 2).
Supplementary Fig. 2.
Hospitalization times of COVID (blue) and pre-COVID period (orange): days between CTA and TAVI (A); days CTA during the TAVI hospitalization (B); Total LoS (C); Post Procedural LoS (D). Abbreviations: COVID, CoronaVirus Disease-19; CTA, Computed Tomography Angiography; TAVI, Transcatheter Aortic Valve Implantation; LoS, Length Of Stay.
In an exploratory analysis including only patients with severe renal impairment at baseline (<30 ml/min/1.73m2), performing pre-procedural CTA the working day before TAVI, was not associated with increased rates of AKI during the COVID-19 pandemic period (25% vs 28.2%, p = 0.75).
3.3. Thirty-day outcomes
Thirty-day outcomes are reported in Table 2 . Thirty-day rates of all-cause death (4.0% vs. 4.5%, p = 0.75) and major adverse cardiovascular events (MACE) (4.0% vs. 6.1%, p = 0.26) were similar between patients treated during the COVID-19 pandemic and pre-pandemic periods. No differences in stroke (1.8 vs. 3.6, p = 0.21), PPI (9.3 vs. 11.3, p = 0.44), MI (0.0 vs. 0.6, p = 0.23) and hospitalization for heart failure (HF) rates (1.8 vs. 1.3, p = 0.66) were reported.
Table 2.
Thirty-day outcomes of pre-COVID and COVID period patients.
| All patients (n = 536) | Pre-COVID period (n = 309) | COVID period (n = 227) | p-Value | |
|---|---|---|---|---|
| All-cause death, n (%) | 23 (4.3) | 14 (4.5) | 9 (4.0) | 0.75 |
| CV death, n (%) | 18 (3.4) | 10 (3.2) | 8 (3.5) | 0.86 |
| Any stroke, n (%) | 15 (2.8) | 11 (3.6) | 4 (1.8) | 0.21 |
| PPI, n (%) | 56 (10.4) | 35 (11.3) | 21 (9.3) | 0.44 |
| MI, n (%) | 2 (0.4) | 2 (0.6) | 0 | 0.23 |
| MACE, n (%) | 28 (5.2) | 19 (6.1) | 9 (4.0) | 0.26 |
| Hospitalization for HF, n(%) | 8 (1.5) | 4 (1.3) | 4 (1.8) | 0.66 |
Abbreviations: COVID, CoronaVirus Disease-19; CV, CardioVascular; HF, Heart Failure; LBBB, Left Bundle Branch Block; MACE, Major Adverse Cardiovascular Events; MI, Myocardial Infarction; PPI, Permanent Pacemaker Implantation; TIA Transient Ischemic Attack.
4. Discussion
In February 2020, first cases of COVID-19 infection were confirmed in Italy. Thereafter, there was an exponential increase of COVID-19 cases, reaching three peaks, the first in March 2020, the second in November 2020 and the last one in March 2021 [7]. During the whole period, total lockdown and dynamic quarantines were carried out all over the country. In order to control the community transmission of COVID-19, the main pandemic goal was to minimize the risk of COVID-19 exposure for all individuals and healthcare providers. Besides, due to the limited resources, there was the necessity to preserve PPE, cardiopulmonary assistance equipment and ICU beds occupation, as well as to rearrange lots of services of healthcare system in general. As a result, access to cardiovascular assistance was limited in the whole Italian territory and relevant issues regarding the priority of cardiac cares and invasive treatments, emerged [8,9].
Due to the poor prognosis of patients affected by severe symptomatic AS, deferring its treatment (either surgical aortic valve replacement (SAVR) or TAVI) by several months would have therefore resulted in thousands of preventable deaths [5]. The European Society of Cardiology (ESC) group proposed criteria for prioritizing treatment of patients with severe AS. Accordingly, patients with reduction in ejection fraction, NYHA class III-IV, or syncope secondary to AS should not be denied to valve replacement. Patients with moderate symptoms should be carefully evaluated and screened for the need of a timely intervention, whereas treatment of patients with mild symptoms might be reasonably postponed by few months. Importantly, the aforementioned document states that the use of a less invasive procedure such as transfemoral TAVI should be offered even to selected low-risk patients, providing the opportunity to minimize ICU and hospital stay [10].
In our center, the TAVI program has not been interrupted during the COVID-19 pandemic, even though we reported a significant decrease in the number of procedures performed from March 2020 to April 2021 due to important restrictions in the access to the hospital mandated by the regional governance. We then remodelled the entire TAVI logistics and work-up to accomplish the changing needs of healthcare systems and face the emerging need of minimizing patients' hospitalization (Supplementary Figs. 3 and 4). Indeed, the staged pre-procedural planning for TAVI was forced in the same hospitalization of the procedure instead of an ad-hoc prior hospital access as in the pre-COVID-19 pandemic period. After the ascertainment of a negative test for COVID-19 infection performed before admission, the ECG-gated CTA was performed the day of hospital admission for TAVI procedure, CTA acquisitions were analyzed the working day after, when the TAVI procedure was scheduled, and concomitant, on-demand case discussion by local Heart Team was carried on just before the beginning of the cath-lab daily planning. Before and during pandemic period, screening of coronary artery disease (CAD) was routinely performed in the same setting of TAVI [11]. In case of severe coronary stenosis (>70%) in proximal segments, percutaneous coronary intervention (PCI) was performed concomitantly to TAVI procedure if there was evidence of myocardial viability at pre-procedural examinations and low PCI complexity was expected.
This strategy allowed to reduce staff and patients potential exposure to COVID-19 infection, and to lower health system overload by performing a single hospitalization for both TAVI workup and procedure and optimizing in-hospital length of stay. Accordingly, a next-day discharge strategy was the standard pathway for uncomplicated TAVI without predictors of developing complete atrio-ventricular block during the pandemic, once again aiming at minimizing in-hospital length of stay [12].
Our analysis showed that the optimized logistics during the pandemic was safe and effective as no differences in mortality and major adverse cardiovascular events were reported compared to pre-pandemic period both in-hospital and at 30 days.
We reported lower rates of life-threatening bleeding and of major vascular complications during the pandemic. The reason behind this finding is probably due to an enhanced collaboration between our local interventional and vascular surgery teams, which set up a new algorithm to treat TAVI candidates with complex vascular accesses. That happened in correspondence with the first COVID-19 outbreak, and led to a better patient selection for either femoral or alternative approaches for TAVI, and subsequent lowering of vascular complication and bleeding rates. Also, these results might be reasonably linked to the greater attention to any vascular complication related to the procedure and the more aggressive strategy adopted to solve them by TAVI operators. In fact, case-by-case evaluation of vascular access closure by multiple digital-subtraction angiographies was carry on in order to timely detect the presence of any sort of small vascular damage, and to promptly treat it with an appropriate endovascular treatment (ballooning or stenting). This allowed to avoid a longer post-procedural care and a higher usage of hospital resources, without reducing the safety of our patients.
Importantly, although performing pre-procedural CTA the day before TAVI procedure could increase the risk of AKI, comparable AKI rates were reported between COVID-19 pandemic and pre-pandemic periods both in the overall population and in the subset of patients with already known renal impairment [13].
The waiting period before TAVI was significantly shorter during COVID-19 period. This was probably the result of either the impact of the new logistics or the refusing of the procedure by patients themselves. Indeed, whilst our TAVI program has been continued during the COVID-19 pandemic, patients refused hospitalization much more often than in the past due to concern about the risk of COVID-19 infection.
Of note, none of 227 TAVI patients tested positive for COVID-19 during the pandemic period. This definitely proved the goodness of the strategies used for minimizing patients' exposure to COVID-19 infection during the in-hospital period.
Our results are concordant with studies carried out in several countries (Israel, United Kingdom, Poland, Holland, France) that did not show significant differences in mortality and procedural outcomes between the pandemic and pre-pandemic periods, thus supporting the possibility to maintain safely the TAVI program by adopting adequate safety measures and pathway during pre, peri- and post-procedural cares [[14], [15], [16], [17]].
The optimization of the TAVI pathway adopted during the COVID-19 pandemic, could be applied to the standard practice after the pandemic period, thus aiming at offering patients a smoother and faster access to TAVI treatment, as well as at decreasing the utilization of hospital resources and therefore minimizing the weight of TAVI treatment on health systems.
5. Limitations
This is a retrospective, single-centre study analysis and therefore it suffered from several limitations. Moreover, outcomes beyond one month after TAVI should be further investigated.
6. Conclusions
The COVID-19 pandemic had a remarkable impact on national healthcare systems worldwide and a number of patients affected by severe AS were forced to postponed their treatment. We showed that the use of an optimized single-hospitalization logistics for TAVI workup and procedure developed during the COVID-19 pandemic, showed to be as safe and effective as the two-stage TAVI pathway previously adopted, allowing the minimization of potential exposure to COVID-19 infection and hospital resources, and shortening times to treatment for severely symptomatic patients. Therefore, such strategy could be reasonably adopted also after the pandemic period.
The following are the supplementary data related to this article.
Supplementary material
Declaration of Competing Interest
None.
Acknowledgements
This study was supported by the Italian Ministry of Health within the call “Ricerca Finalizzata 2018” (code GR-2018-12365438).
References
- 1.Zhu N., Zhang D., Wang W., Li X., Yang B., Song J., Zhao X., Huang B., Shi W., Lu R., Niu P., Zhan F., Ma X., Wang D., Xu W., Wu G., Gao G.F., Tan W. A novel coronavirus from patients with pneumonia in China, 2019. N. Engl. J. Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vecchio S., Fileti L., Reggi A., Moschini C., Lorenzetti S., Rubboli A. Impact of the COVID-19 pandemic on admissions for acute coronary syndrome: review of the literature and single-center experience. G. Ital. Cardiol. (Rome) 2020;21:502–508. doi: 10.1714/3386.33635. [DOI] [PubMed] [Google Scholar]
- 3.Nourazari S., Davis S.R., Granovsky R., Austin R., Straff D.J., Joseph J.W., Sanchez L.D. Decreased hospital admissions through emergency departments during the COVID-19 pandemic. Am. J. Emerg. Med. 2021;42:203–210. doi: 10.1016/j.ajem.2020.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barbanti M., van Mourik M.S., Spence M.S., Icovelli F., Martinelli G.L., Muir D.F., Saia F., Bortone A.S., Densem C.G., van der Kley F., Bramlage P., Vis M., Tamburino C. Optimising patient discharge management after transfemoral transcatheter aortic valve implantation: the multicentre European FAST-TAVI trial. EuroIntervention. 2019;15:147–154. doi: 10.4244/EIJ-D-18-01197. [DOI] [PubMed] [Google Scholar]
- 5.Otto C.M., Prendergast B. Aortic-valve stenosis — from patients at risk to severe valve obstruction. N. Engl. J. Med. 2014;371:744–756. doi: 10.1056/NEJMra1313875. [DOI] [PubMed] [Google Scholar]
- 6.Kappetein A.P., Head S.J., Généreux P., Piazza N., van Mieghem N.M., Blackstone E.H., Brott T.G., Cohen D.J., Cutlip D.E., van Es G.-A., Hahn R.T., Kirtane A.J., Krucoff M.W., Kodali S., Mack M.J., Mehran R., Rodés-Cabau J., Vranckx P., Webb J.G., Windecker S., Serruys P.W., Leon M.B. Updated standardized endpoint definitions for transcatheter aortic valve implantation. J. Am. Coll. Cardiol. 2012;60:1438–1454. doi: 10.1016/j.jacc.2012.09.001. [DOI] [PubMed] [Google Scholar]
- 7.Worldometer COVID-19 Data, (n.d.). https://www.worldometers.info/coronavirus/country/italy/.
- 8.Sofi F., Dinu M., Reboldi G., Stracci F., Pedretti R.F.E., Valente S., Gensini G., Gibson C.M., Ambrosio G. Worldwide differences of hospitalization for ST-segment elevation myocardial infarction during COVID-19: a systematic review and meta-analysis. Int. J. Cardiol. 2022;347:89–96. doi: 10.1016/j.ijcard.2021.10.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Onder G., Olimpieri P.P., Celant S., Di Lenarda A., Ambrosio G., Reboldi G., Gensini G., Colatrella A., Palmer K., Gabrielli D., Russo P. Under-prescription of direct oral anticoagulants for treatment of non-valvular atrial fibrillation and venous thromboembolism in the COVID-19 lockdown period. Eur. J. Prev. Cardiol. 2021 doi: 10.1093/eurjpc/zwab096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.ESC Guidance for the Diagnosis and Management of CV Disease during the COVID-19 Pandemic. 2020. [Google Scholar]
- 11.Barbanti M., Todaro D., Costa G., Pilato G., Picci A., Gulino S., Capranzano P., La Spina K., Di Simone E., D’Arrigo P., Deste W., Indelicato A., Cannata S., Giannazzo D., Immè S., Tamburino C., Patanè M., Buccheri S., Capodanno D., Sgroi C., Tamburino C. Optimized screening of coronary artery disease with invasive coronary angiography and ad hoc percutaneous coronary intervention during transcatheter aortic valve replacement. Circ. Cardiovasc. Interv. 2017;10 doi: 10.1161/CIRCINTERVENTIONS.117.005234. [DOI] [PubMed] [Google Scholar]
- 12.Costa G., Barbanti M., Picci A., Todaro D., La Spina K., Di Simone E., D’Arrigo P., Criscione E., Valvo R., Reddavid C., Deste W., Sgroi C., Tamburino T. Predictors and safety of next-day discharge in unselected patients undergoing transfemoral transcatheter aortic valve implantation. EuroIntervention. 2020 doi: 10.4244/EIJ-D-19-01080. [DOI] [PubMed] [Google Scholar]
- 13.Barbanti M., Gulino S., Capranzano P., Immè S., Sgroi C., Tamburino C., Ohno Y., Attizzani G.F., Patanè M., Sicuso R., Pilato G., Di Landro A., Todaro D., Di Simone E., Picci A., Giannetto G., Costa G., Deste W., Giannazzo D., Grasso C., Capodanno D., Tamburino C. Acute kidney injury with the RenalGuard system in patients undergoing transcatheter aortic valve replacement: the PROTECT-TAVI trial (PROphylactic effecT of furosEmide-induCed diuresis with matched isotonic intravenous hydraTion in Transcatheter aortic Va) JACC Cardiovasc. Interv. 2015;8 doi: 10.1016/j.jcin.2015.07.012. [DOI] [PubMed] [Google Scholar]
- 14.Valdebenito M., Massalha E., Barbash I.M., Maor E., Fefer P., Guetta V., Segev A. Transcatheter aortic valve implantation during the COVID-19 pandemic. Am. J. Cardiol. 2021;145:97–101. doi: 10.1016/j.amjcard.2020.12.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Adlam D., Chan N., Baron J., Kovac J. Aortic stenosis in the time of <scp>COVID</scp> −19: development and outcomes of a rapid turnaround <scp>TAVI</scp> service. Catheter. Cardiovasc. Interv. 2021 doi: 10.1002/ccd.29550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Perek B., Olasinska-Wisniewska A., Misterski M., Puslecki M., Grygier M., Buczkowski P., Lesiak M., Stankowski T., Szarpak L., Ruetzler K., Turan O., Jemielity M. How the COVID-19 pandemic changed treatment of severe aortic stenosis: a single cardiac center experience. J. Thorac. Dis. 2021;13:906–917. doi: 10.21037/jtd-20-3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rooijakkers M.J.P., Li W.W.L., Wollersheim L.W.L.M., Geuzebroek G.S.C., Gehlmann H., Garsse L.A.F.M., Wely M.H., Verkroost M.W.A., Morshuis W.J., Wertheim H., Royen N. Transcatheter aortic valve replacement during the COVID-19 pandemic—a Dutch single-center analysis. J. Card. Surg. 2021;36:48–55. doi: 10.1111/jocs.15123. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material