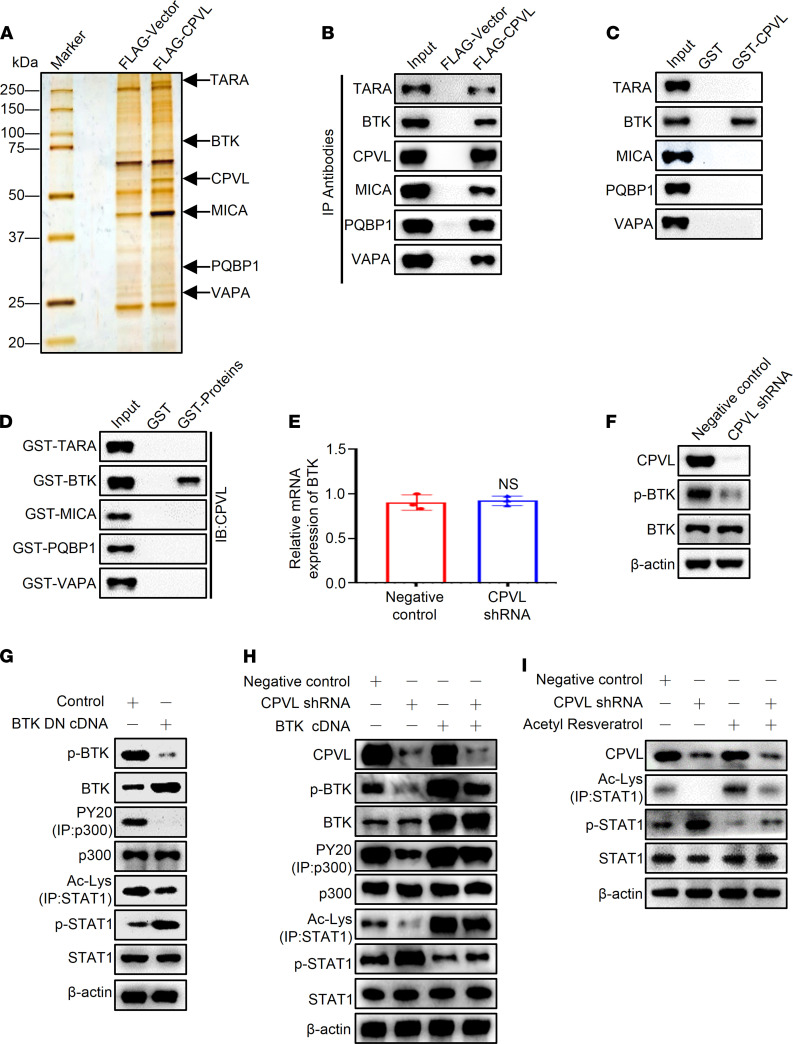
Figure 7. CPVL physically interacts with BTK to regulate the STAT1 phosphorylation through p300-mediated STAT1 acetylation.
(A) Immunoaffinity purification of CPVL-containing protein complex. Cellular extracts from U251 cells stably expressing FLAG vector or FLAG-CPVL were immunopurified with anti-FLAG affinity columns and eluted with FLAG peptide. These eluates were resolved by SDS-PAGE and were silver stained. (B) IP of whole-cell lysates from U251 cells followed by IB with antibodies against the indicated proteins. (C) GST pull-down assays with GST-fused CPVL and in vitro transcribed/translated TARA, BTK, MICA, PQBP1, or VAPA as indicated. (D) GST pull-down assays with the indicated GST-fused proteins and in vitro transcribed/translated CPVL. (E and F) U251 cells were transfected with a control shRNA or CPVL shRNA. The mRNA level of BTK and the protein levels of CPVL, BTK, and p-BTK were measured. (G) U251 cells were treated with control cDNA or BTK DN cDNA, and the protein levels of p-BTK, BTK, p300, PY20 (IP: p300), Ac-Lys (IP: STAT1), p-STAT1, and STAT1 were measured by Western blotting. (H) Rescue experiments were used to investigate whether the biological function of CPVL was mediated by regulating phosphorylation of BTK. U251 cells were transfected with CPVL shRNA or CPVL shRNA plus BTK cDNA compared with the control cells, and the protein levels of CPVL, p-BTK, BTK, p300, PY20 (IP: p300), Ac-Lys (IP: STAT1), p-STAT1, and STAT1 were measured by Western blotting. (I) Rescue experiments were used to investigate the STAT1 phosphorylation in CPVL-silenced U251 cells treated with acetylation activator, acetyl resveratrol, compared with the control cells, and the protein levels of CPVL, STAT1, Ac-Lys (IP: STAT1), and p-STAT1 were measured by Western blotting. All experiments were repeated 3 times. β-Actin was used as a loading control. Bar graph data are presented as mean ± SD. Two-tailed Student’s t test analyses were performed.