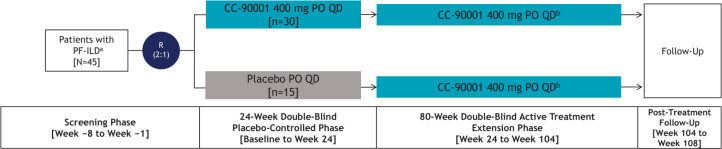
Figure 2.
PF-ILD exploratory substudy design. a Eligible patients include those not receiving pirfenidone who were screened and did not receive a confirmed IPF diagnosis following central reading of HRCT and lung biopsy (if obtained). b The decision to receive additional treatments for pulmonary fibrosis (eg, pirfenidone) during the up to 80-week, active treatment extension phase is at the discretion of the investigator. HRCT, high-resolution CT; IPF, idiopathic pulmonary fibrosis; PF-ILD, progressive fibrotic interstitial lung disease; PO, oral; QD, one time per day; R, randomisation.