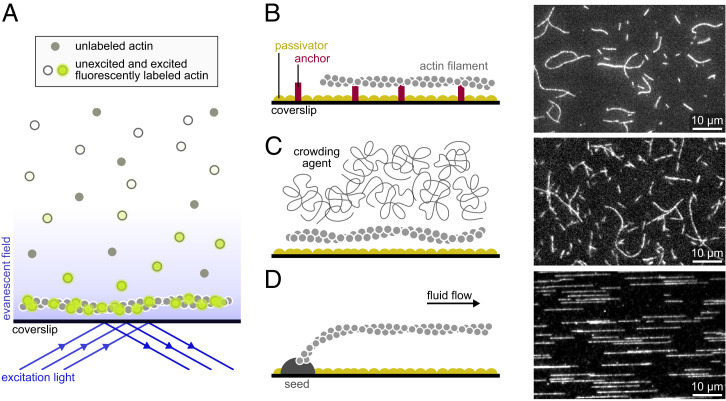
Fig. 1.
Main single-filament techniques used to study actin dynamics. (A) In TIRF microscopy, a shallow region of the sample, a few hundred nanometers above the coverslip, is illuminated by the evanescent wave resulting from the total reflection of the incoming light. Note that if the concentration of labeled proteins is low, TIRF may not be necessary, and epifluorescence can be used. (B–D) Actin filaments can be maintained close to the surface by specific anchors (A), by a crowding agent present in solution (B), or by a microfluidic flow while they are anchored by one end only (here, via a seed; C). (B, Right; C, Right; and D, Right) Actin filaments (10% labeled with AlexaFluor-488 and 1% biotinylated actin subunits on surface lysines) anchored to a streptavidin functionalized coverslip (A), actin filaments (10% labeled with AlexaFluor-568) with 0.3% methylcellulose (4,000 cP at 2%; B), and actin filaments (10% labeled with AlexaFluor-488) polymerized from spectrin-actin seeds inside a microfluidic chamber (C). All filaments are made with rabbit alpha-skeletal actin, imaged in the same buffer (5 mM Tris⋅HCl, pH 7.4, 50 mM KCl, 1 mM MgCl2, 0.2 mM EGTA, 0.2 mM ATP, 10 mM DTT, 1 mM DABCO). EGTA, ethylene glycol tetraacetic acid; DTT, dithiothreitol; DABCO, diazabicyclooctane.