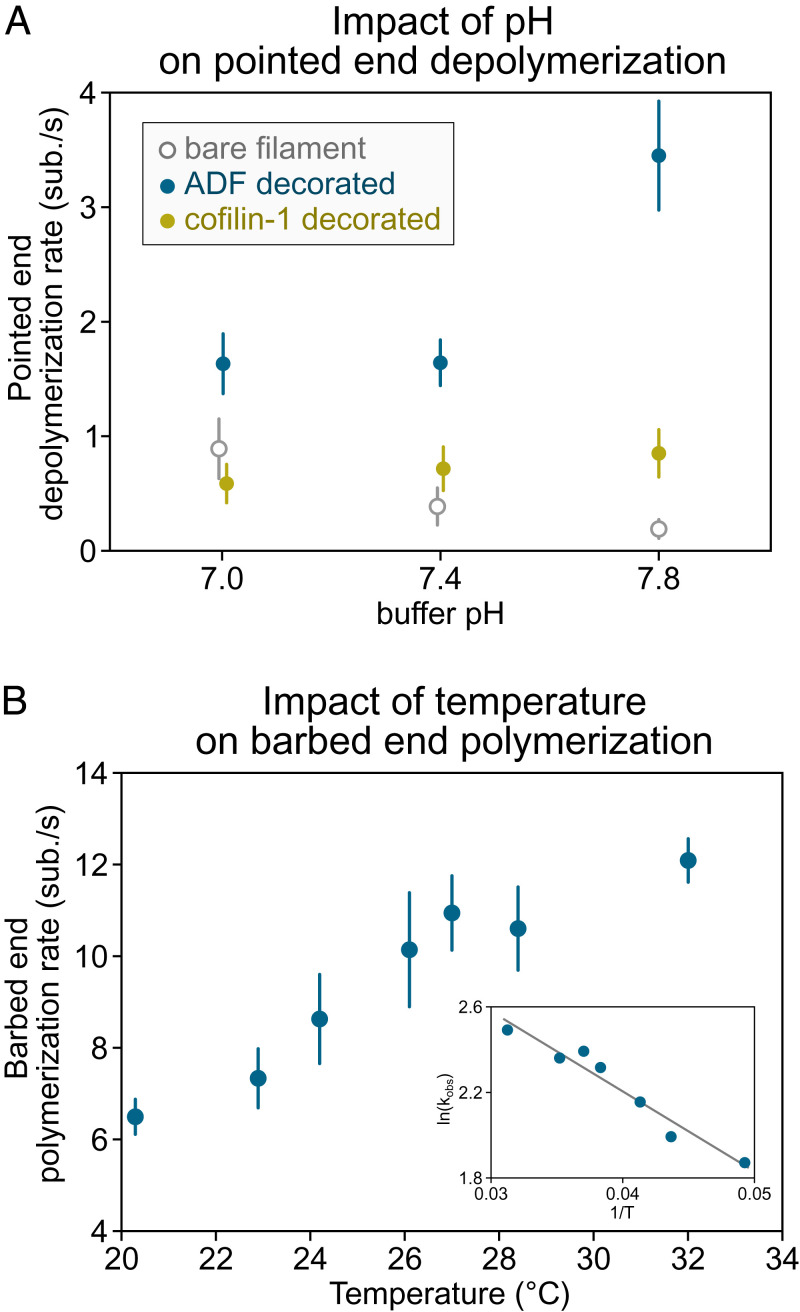
Fig. 4.
Impact of pH and temperature on reaction rates. (A) Depolymerization of actin filament pointed ends (15% labeled with AlexaFluor-568 on surface lysines) at different buffer pH (5 mM Tris⋅HCl, 50 mM KCl, 1 mM MgCl2, 0.4 mM CaCl2, 0.2 mM EGTA, 0.2 mM ATP, 10 mM DTT, 1 mM DABCO) supplemented with 1 to 2 µM ADF or cofilin-1 as indicated. Filaments are attached to the coverslip at their barbed end by gelsolin in a microfluidics chamber. Depolymerization of bare and ADF/cofilin-decorated filaments was recorded for up to 30 min at one frame every 30 to 60 s. Each data point represents the mean and SD over 14 to 36 filaments (from one experiment). Data adapted from ref. 99 with permission from the American Chemical Society (ACS), further permission should be directed to the ACS. (B) Elongation of the actin filament barbed end from anchored spectrin-actin seeds in a microfluidics flow, at different temperatures, exposed to 1 µM Mg-ATP-actin (10% AlexaFluor-488 labeled on surface lysines) in buffer (5 mM Tris⋅HCl, pH 7.4, 50 mM KCl, 1 mM MgCl2, 0.2 mM EGTA, 0.2 mM ATP, 10 mM DTT, 1 mM DABCO). Elongation was recorded for 5 min at one frame every 10 s, and n > 30 filaments were analyzed for each condition. (Inset) The slope of the linear relation of log(elongation rate) as a function of the inverse of the temperature (Arrhenius plot) allows us to estimate the free energy associated with actin monomer addition at the barbed end to be 37.8 kJ/mol (9.03 kcal/mol), similar to the value reported by Drenckhahn and Pollard (100) using EM. Data can be found in Dataset S1. ADF, actin depolymerizing factor; EGTA, ethylene glycol tetraacetic acid; DTT, dithiothreitol; DABCO, diazabicyclooctane.