Figure 1.
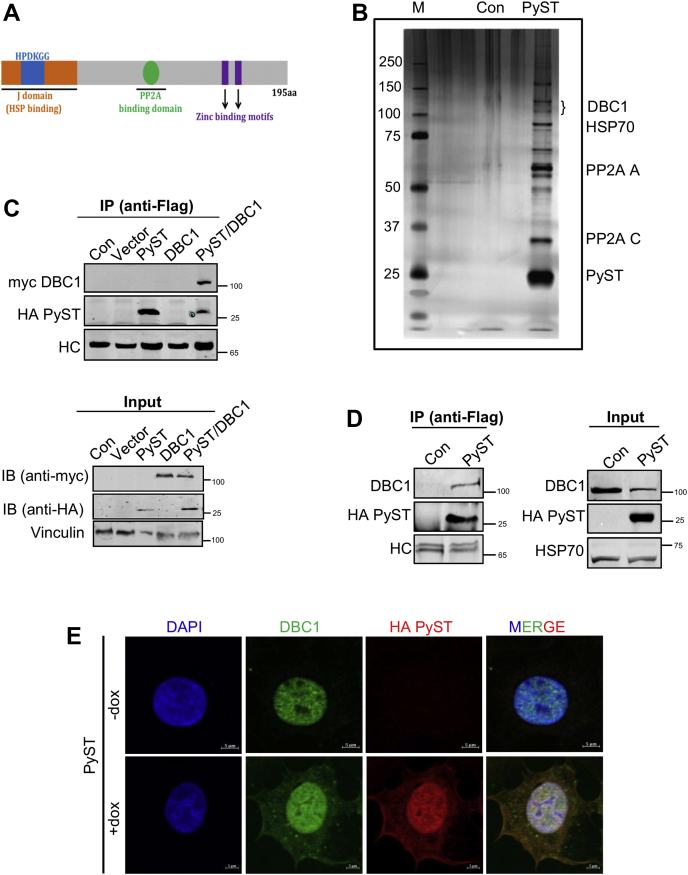
Polyoma small T (PyST) interacts with DBC1.A, schematic representation of polyoma virus small T antigen and its structural features, showing an N-terminal J domain and a C-terminal PP2A-binding domain. B, PyST-associated complexes isolated from pTREX-PyST-HA-FLAG cell lines by tandem affinity purification (TAP) using consecutive anti-FLAG and anti-HA affinity matrices were run on an SDS-PAGE and visualized by silver staining. C, 293T cells were cotransfected with pcDNA3-PyST-HA-Flag (1.5 μg), myc-DBC1 (1.5 μg), and empty vector (pCDNA3.1, 1.5 μg). PyST-HA-Flag was immunoprecipitated with anti-Flag antibody and subjected to SDS-PAGE and immunoblotting (IB) with anti-myc and anti-HA antibodies, as indicated. D, immunoprecipitation of endogenous DBC1 from 293T cells. These cells were transfected with pcDNA3-PyST-HA-Flag (1.5 μg). PyST was immunoprecipitated using anti-Flag antibody. Endogenous DBC1 was detected using anti-DBC1 antibody. In C and D immunoprecipitate panels, HC designates heavy chain of IgG. E, colocalization results of PyST (red) and DBC1 (green) using immunofluorescence microscopy obtained at about 24 h of doxycycline addition. Nuclei were stained with DAPI (63× magnification; the scale bar represents 5 μm). The results are representative of three independent experiments. DBC1, deleted in breast cancer 1; HSP, heat shock protein; PP2A, protein phosphatase 2A.