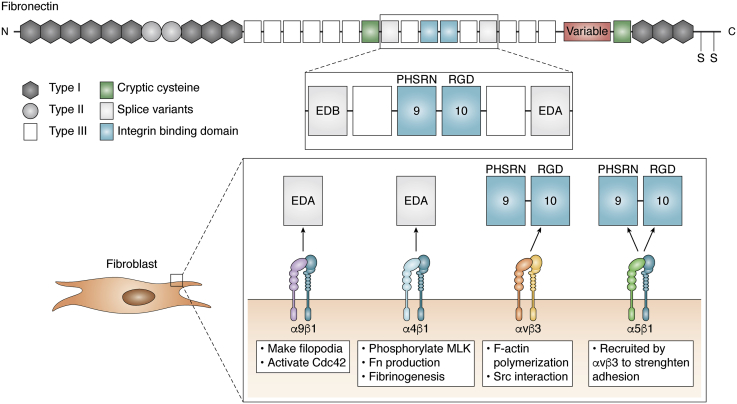
Figure 2.
Fibronectin structure and fibroblast integrins that bind it.Top, schematic of a single 220 kDa subunit of fibronectin. Type III repeats making up the integrin-binding domain are in blue, extra domain type III repeats that are part of the cellular fibronectin (cFn) isoform are in gray. Green type III (7th and 15th) domains where glutathionylation may occur under conditions that unfold the protein domains. Bottom, the fibroblast integrins binding Fn are the following: α9β1 and α4β1 bind the extra domain A present in cell secreted Fn, whereas αvβ3 and α5β1 bind the canonical integrin-binding domain. While αvβ3 binds the RGD motif on the 10th type III repeat (and other proteins), integrin α5β1 requires the “synergy” PHSRN peptide sequence on the ninth type III to be in close proximity. Cell-generated forces on Fn fibers can unfold the type III repeats, increasing the distance between RGD and the synergy site, inhibiting α5β1 engagement. Under those conditions, only αvβ3 can properly bind the integrin-binding domain of Fn. This phenomenon, first predicted via steered molecular dynamics, has been named “integrin switch.”