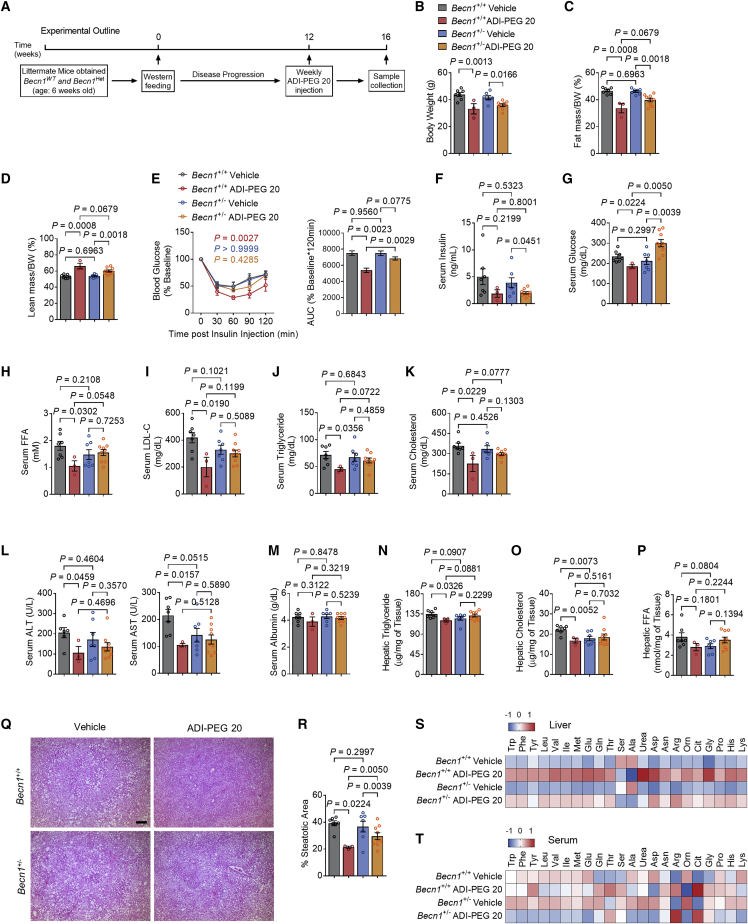
Figure 6.
Whole-body Becn1 Het abolishes the therapeutic effects of ADI-PEG 20 in WD-fed mice
(A) Schematic of experimental design used to test the role of ADI-PEG 20 in Becn1Het WD-fed mice.
(B–D) Body weight (B), body fat (C), and lean mass (D) percentage of composition of vehicle- and ADI-PEG 20-treated Becn1 Het mice (n = 7, 3, 7, and 9 mice per group).
(E) Intraperitoneal tolerance tests for insulin (ITT).
(F and G) Serum insulin (F) and serum glucose (G) in vehicle- and ADI-PEG 20-treated Becn1Het mice.
(H–K) Serum non-esterified fatty acid (H), low-density lipoprotein cholesterol (I), triglyceride (J), and cholesterol (K) in vehicle- and ADI-PEG 20-treated Becn1 Het mice.
(L and M) Serum ALT (L) and serum albumin (M) contents in vehicle- and ADI-PEG 20-treated Becn1 Het mice.
(N–P) Triglyceride (N), cholesterol (O), and non-esterified fatty acid (P) contents in the livers of vehicle- and ADI-PEG 20-treated Becn1 Het mice.
(Q and R) Liver sections stained with hematoxylin and eosin (H&E, Q) with steatotic (e.g., aparenchymal space) quantified (R). Scale bars, 100 μm.
(S and T) Targeted metabolomic analysis of liver (S) and serum (T) amino acids and urea cycle intermediaries from vehicle- and ADI-PEG 20-treated Becn1Het mice.
Data represented in mean ± SEM. Each data point represents an individual animal. Exact p values are shown. Statistical significance was determined using two-way ANOVA in (E). Unpaired two-tailed Student’s t test was used in (B)–(D) and (F)–(R).