Fig. 1. Generation of clinical-grade iPSC-RPE cells.
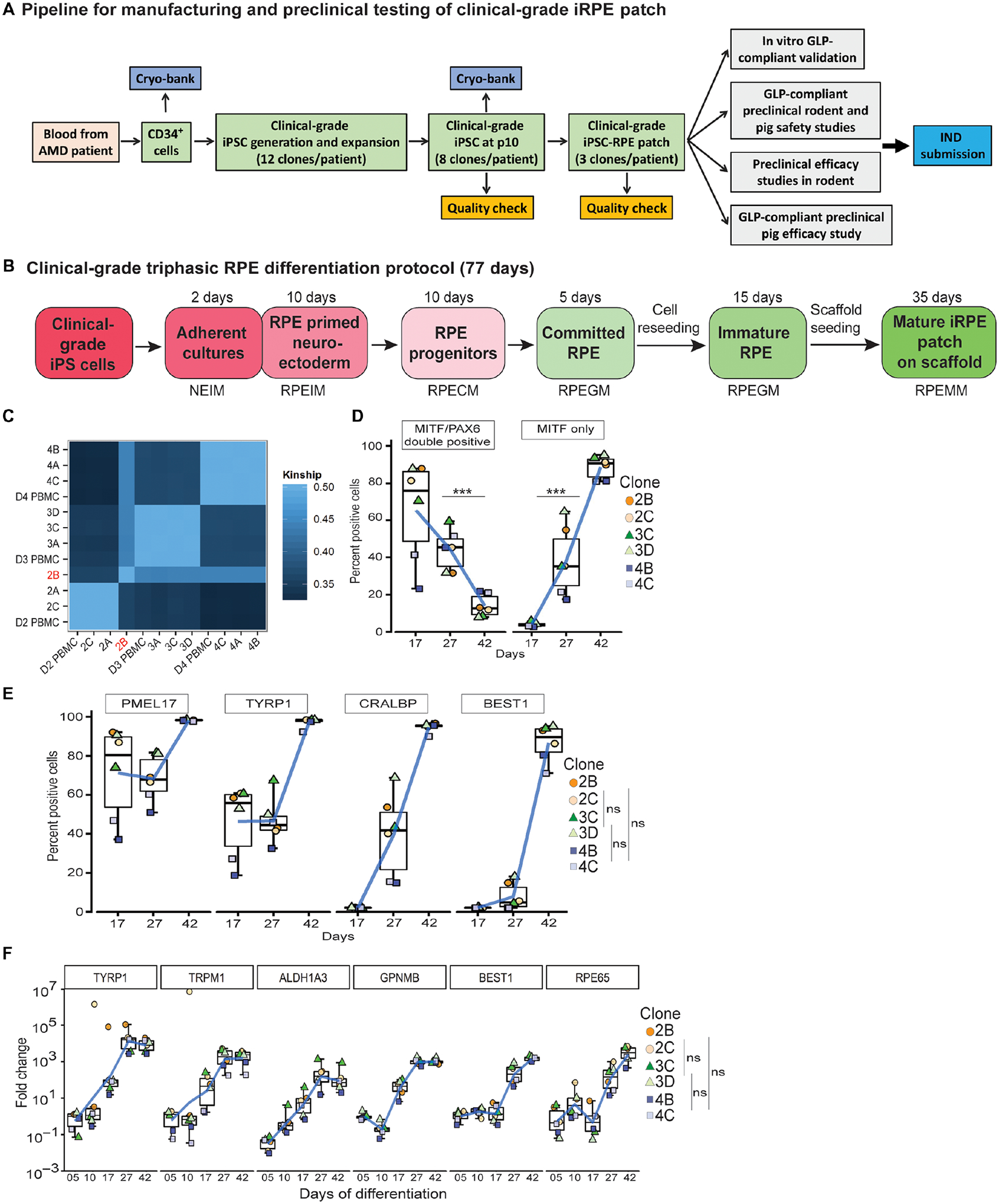
(A) Workflow illustrating a pipeline to manufacture and test autologous clinical-grade iPSC-RPE-patches with the goal of filing a phase I clinical trial Investigational New Drug (IND)-application to the FDA. (B) Time-line of clinical-grade iRPE differentiation. Clinical-grade iRPE differentiation takes 77 days, is initiated with monolayer iPSCs and performed using xeno-free reagents. Neuro Ectoderm Induction Medium (NEIM); RPE Induction Medium (RPEIM); RPE Commitment Medium (RPECM); RPE Growth Medium (RPEGM); RPE Maturation Medium (RPEMM). (C) Coding-region sequencing of 223 oncogenes at 2000x depth for all nine clinical-grade AMD iPSC clones. (D, E) Flow cytometry analysis of clinical-grade iPSC-RPE derived from three AMD patients, performed at the RPE progenitor stage day (D)17, RPE-commitment stage (D27), and immature RPE-stage (D42) (n=6). Analysis of variance (ANOVA) was performed to determine changes in percent positive cells; ***p=0.0001 for PAX6/MITF and ***p=8.9×10−14 for MITF; Dunn’s test was performed for pair-wise comparisons; p-values: 2B/2C- 3C/3D=0.909; 2B/2C-4B/4C=0.400; 3C/3D-4B/4C=0.319 (F) RPE-specific gene expression from D5–D42 of clinical-grade iPSC-RPE differentiation (n=6). Dunn’s test was performed for pair-wise comparisons; p-values: 2B/2C- 3C/3D=0.721; 2B/2C- 4B/4C=0.719; 3C/3D-4B/4C=0.999.
