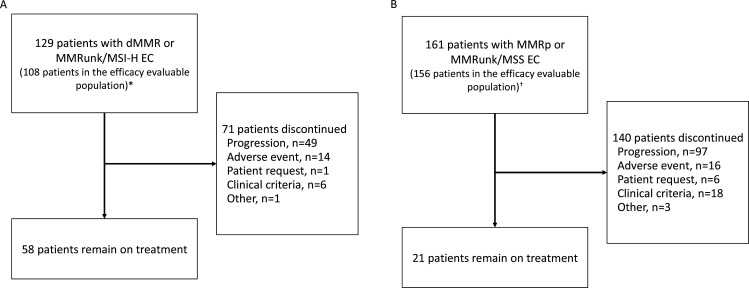
Figure 1.
Enrollment and outcomes. *Twenty-one patients had no measurable disease by BICR at baseline (n=9) or had insufficient follow-up time (<6 months, n=12) and were excluded from this interim analysis efficacy-evaluable population; three patients with <6 months of follow-up time who had discontinued treatment (each with a best response of not evaluable) were included in the efficacy-evaluable population. †Sixteen patients had no measurable disease by BICR at baseline or had insufficient follow-up time (<6 months) and were excluded from this interim analysis efficacy-evaluable population. BICR, blinded independent central review; dMMR, mismatch repair deficient; EC, endometrial cancer; MMR, mismatch repair proficient; MMRun, mismatch repair unknown; MSI-H, microsatellite instability-high; MSS, microsatellite stable.