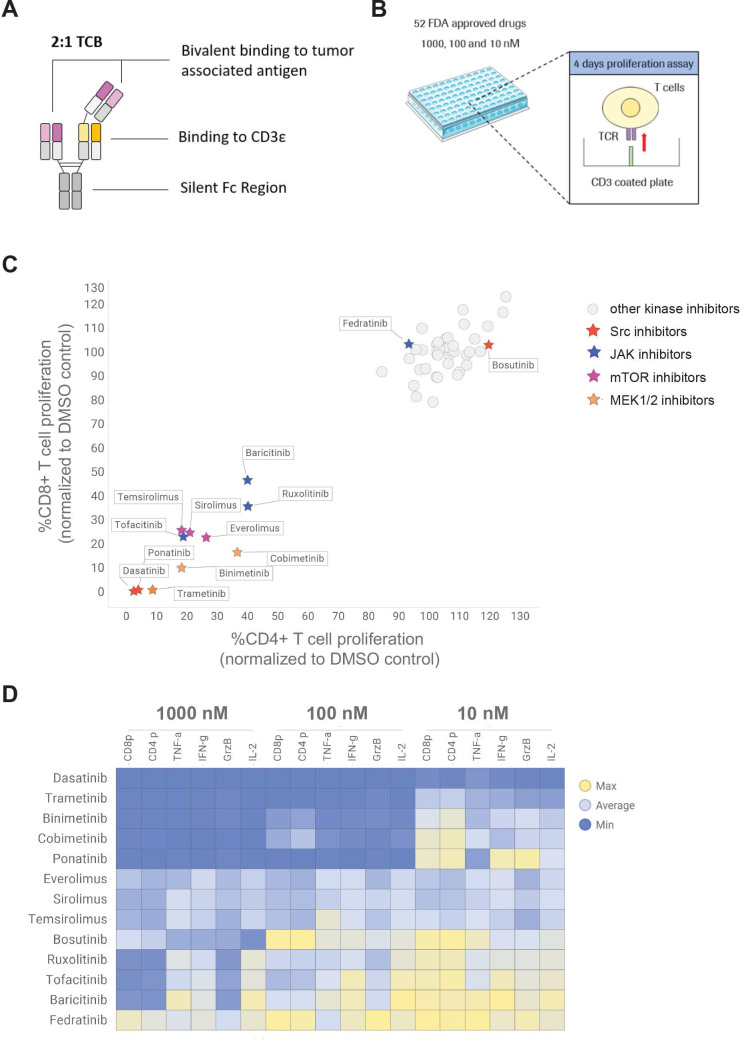
Figure 1.
High throughput screening of 52 FDA-approved kinase inhibitor to identify candidates reducing TCB-induced T cell proliferation and cytokine release. (A) TCBs are fully humanized IgG1 antibodies providing bivalent binding to the tumor associated antigen and monovalent binding to the CD3ε of T-cell receptor on T cells (2+1 format). (B) CTV-labeled pan T cells were stimulated on CD3 coated plate in the absence and presence of 10, 100 and 1000 nM of each kinase inhibitor to mimic the TCB stimulation. (C) The dilution peaks of the CTV dye were measured by flow cytometry at 96 hours to evaluate the effect of the different kinase inhibitors on CD4+ and CD8+ T cell proliferation. The proliferation of CD4+ and CD8+ cells was normalized to proliferation of untreated T cells (DMSO control). mTOR, JAK, Src and MEK inhibitors were identified as hits of the screen. (D) The effects of escalating concentrations of the selected mTOR, JAK, Src and MEK inhibitor candidates on TNF-α, IL-2, granzyme-B (GrzB), IFN-γ and CD4+ (CD4 p) and CD8+ (CD8 p) proliferation are depicted in a heat map. The levels of TNF-α, IL-2, GrzB and IFN-γ were measured in the supernatants by CBA (24 hours) and normalized to untreated T cells. Median of technical triplicates, one donor. CBA, cytometric bead array; CTV, cell trace violet; DMSO, dimethyl sulfoxide; FDA, Food and Drug Administration; IFN, interferon; IL, interleukin; TCB, T cell bispecific antibody; TCR, T cell receptor; TNF, tumor necrosis factor.