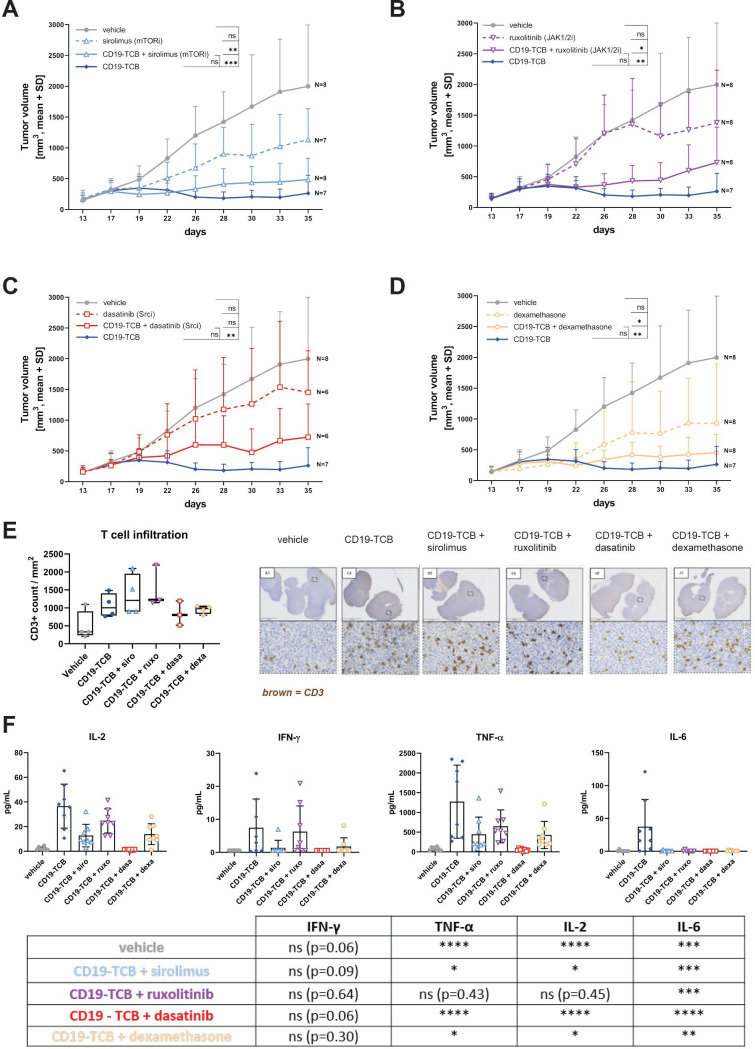
Figure 5.
mTOR and JAK inhibitors do not inhibit CD19-TCB antitumor efficacy in vivo. Humanized NSG mice were engrafted with a lymphoma PDX (5 million cells, s.c.). When tumors reached 200 mm3, mice were randomized in groups of eight or seven based on their tumor size. They were treated with vehicle (intravenously), 5 mg/kg sirolimus (p.o.), 30 mg/kg ruxolitinib (p.o.), 20 mg/kg dasatinib (p.o.), two times 1 mg/kg, 0.5 mg/kg, four times 0.25 mg/kg dexamethasone (p.o.) alone or in combination with 0.5 mg/kg CD19-TCB (intravenously), 0.5 mg/kg CD19-TCB (intravenously) as a monotherapy. (A–D) Tumor growth curves were plotted from tumor volumes measured using a Caliper, mean of n=6–8 mice+SD with *p≤0.05, **p≤0.01, ***p≤0.001 by one-way ANOVA (Kruskal-Wallis test). (E) Trends of CD3 counts in the tumors at experiment termination. Tumor sections were immunohistochemically stained with anti-CD3 (brown) and nuclei were counterstained with hematoxylin. CD3+ T cells were quantified in the different sections with the Visiopharm software. (F) The levels of IL-2, IFN-γ, IL-6 and TNF-α in the serum of the mice collected 6 hours post first infusion with CD19-TCB. The statistical differences to CD19-TCB treatment are summarized in the table. Mean of n=6–8 mice+SD with *p≤0.05, **p≤0.01, ***p≤0.001 by one-way ANOVA (Kruskal-Wallis test). ANOVA, analysis of variance; IFN, interferon; IL, interleukin; PDX, patient-derived xenograft; s.c., subcutaneously; p.o., orally; TCB, T cell bispecific antibody; TNF, tumor necrosis factor.