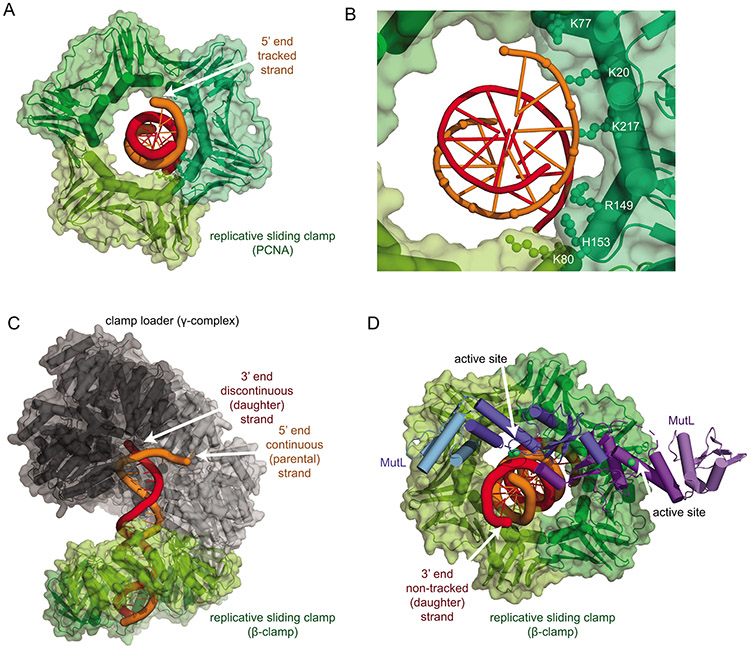
Figure 6. The strand-specific clamp diffusion model for strand-specific cleavage.
A. Structure of the human PCNA trimer (green surface and cartoon) bound to a double-stranded DNA fragment with the tracked strand in orange (PDB ID 6gis (106)). B. Detail of the interactions of the phosphates of the tracked DNA strand with positively charged residues primarily in one PCNA subunit. C. The bacteria β-clamp (green) is loaded onto the continuous (parental; orange) strand of a DNA duplex by the clamp loader (subunits black to grey) as shown in Figure 5A, orienting the clamps to track along the parental strand by strand-specific diffusion. D. The β-clamp (green) interaction with the regulatory subdomain of the MutL C-terminus (light blue) places the active site of the molecule (dark blue) in an appropriate position to cleave the non-tracked, daughter DNA strand (red). This model, together with strand-specific loading shown in panel C, explains how nicks, the replicative sliding clamp, and MutL homologs can mediate strand specificity. The model was generated using the structure of the B. subtilis β-clamp fused to the MutL regulatory subdomain (PDB ID 6e8d (100)), the B. subtilis MutL C-terminal domain (PDB ID 3kdk (64)), and the E. coli β-clamp DNA complex (PDB ID 3bep (111)).