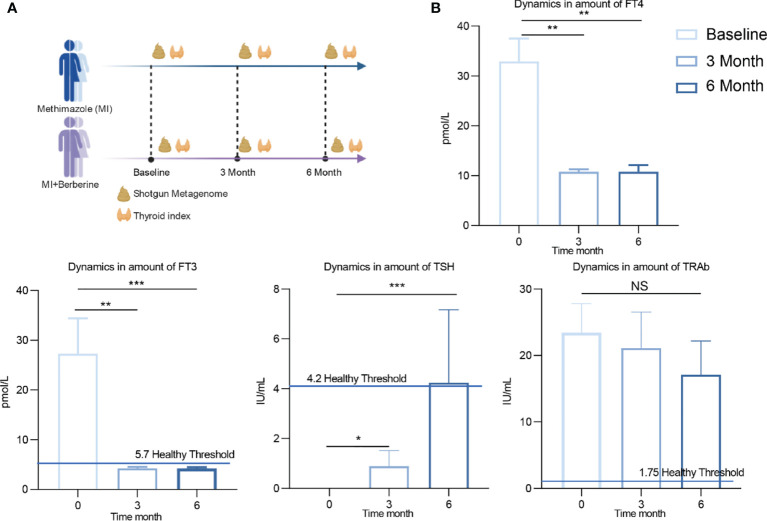
Figure 1.
Experimental design and changes of clinical indexes after treatment with methimidazole. (A) The study was divided into two groups, the methimazole treatment group (n=8) and the combined methimazole and berberine treatment group (n=10) both groups were treated for 6 months. Serum and stool samples were collected at baseline, 3 months and 6 months for measurement of thyroid parameters and shotgun metagenomic sequencing, respectively. (B) Concentrations of thyroid indicators such as free triiodothyronine (FT3), free thyroxine (FT4), thyroid stimulating hormone (TSH) and thyroid stimulating hormone receptor antibody (TRAb) at baseline, 3 months of treatment and 6 months of treatment. The differences in the levels of thyroid indicators between the two groups were compared using the Kruskal-Wallis test, and the differences between the two groups were statistically significant. Asterisks indicate significant differences between baseline and endpoints in the corresponding groups (***P < 0.001, **P < 0.01, *P < 0.05) NS means there is no significant difference between the two groups, error line means ± SEM values.