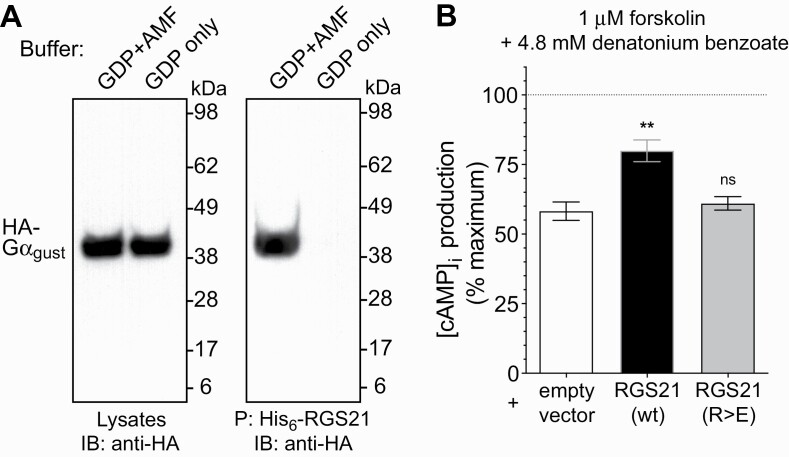
Fig. 2.
RGS21 binds gustducin-α and reduces bitterant signaling in a bitterant-responsive cultured cell line. (A) COS7 cell monolayers were transiently transfected with plasmid DNA encoding gustducin-α tagged with an HA epitope (“HA-Gα gust”). Forty-eight hours post transfection, cell monolayers were lysed in buffer containing GDP, Mg2+ and AlF4– (“AMF”) or GDP alone, as indicated. Clarified cell lysates were incubated with 10 μg of purified His6-RGS21 protein at 4°C overnight with NTA-agarose. Samples were centrifuged, agarose beads washed four times in lysis buffer, and precipitated proteins (“P”:) eluted by boiling for 5 minutes in loading buffer prior to being resolved by SDS–PAGE electrophoresis on the basis of molecular weight (“kDa” = kiloDalton), transferred to a nitrocellulose membrane, and detected using anti-HA antibody immunoblotting (“IB”:) and chemiluminescence. (B) Overexpression of wild-type RGS21, but not a loss-of-function, point mutant RGS21, leads to inhibition of bitterant signaling-induced reduction of cAMP levels. Cultures of the 16HBE cell line were transiently transfected with GloSensor cAMP biosensor cDNA and expression plasmids containing open reading frames for wild-type (“wt”) RGS21, or Arg-126-to-Glu point mutated (“R>E”) RGS21, or empty pcDNA3.1 vector, as indicated. Inhibition of forskolin-stimulated cAMP production (“100% maximum”; dotted line) by treatment with indicated concentration of the bitterant denatonium benzoate (i.e. EC50 previously established as per Supplementary Fig. S1) was determined 24 hr post-transfection by detection of GloSensor-dependent luminescence. Asterisks denote statistically significant differences as determined by one-way ANOVA with Bonferroni’s post-test: **, P < 0.01; ns, not significant (P >> 0.05).