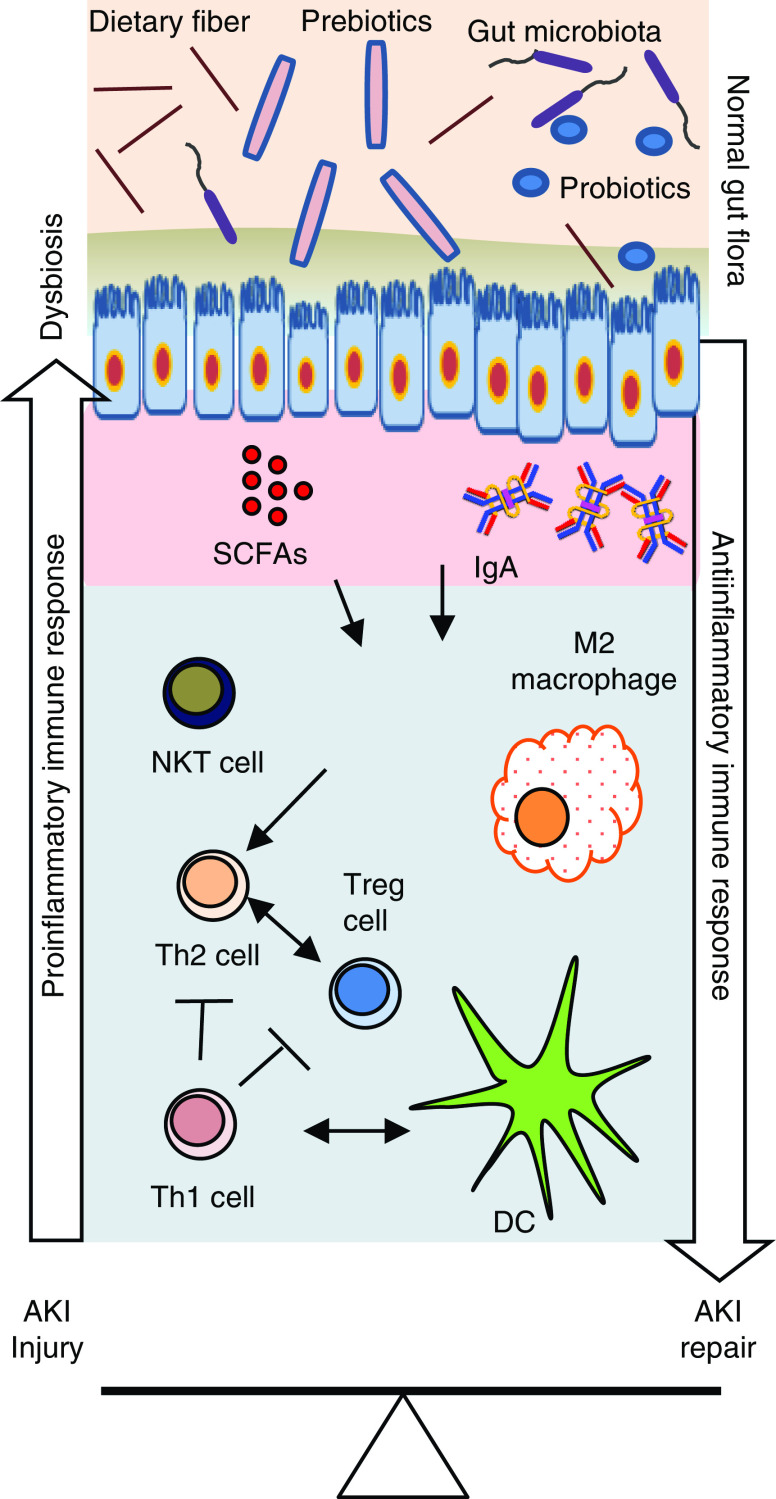
Figure 2.
An overview of gut microbiota–immune cell interactions in the kidney affecting injury and repair processes after AKI. Gut microbiota produce short-chain fatty acids (SCFAs), such as acetate, propionate, and butyrate, that interact with multiple G protein–coupled receptors on kidney epithelial cells. Similar interactions are likely involved in immune cells that modulate their number, immune function, and metabolism in the kidney. Normal gut microbiota promote the anti-inflammatory milieu by increasing T helper 2 (Th2) cell, regulatory T (Treg) cell, and M2 macrophage populations that protect kidneys from AKI. However, AKI-induced dysbiosis and translocation of bacterial products across the leaky intestine promote a proinflammatory immune environment, such as increased Th1 cells, M1 macrophages, and activated dendritic cells (DCs). Activated DCs secrete proinflammatory cytokines, such as IL-12 and IL-6, and skew the differentiation of naive CD4 T cells and maturation of B cells. Th17-inducing bacteria may promote Th17 immunity via IL-17A/IL-17F induction, which may involve signaling mediated by the Toll-like receptor ligands. Additionally, IgA produced by plasma cells residing in the gut epithelium modulates response to colonization by specific commensal bacteria. SCFAs also regulate cytokine expression in T cells and generation of Treg cells through histone deacetylase inhibition. Therapeutic and supplemental use of pre-, pro-, and postbiotics could be helpful in normalizing bacterial composition in the gut and in protecting the kidneys from AKI. NKT, natural killer T cell.