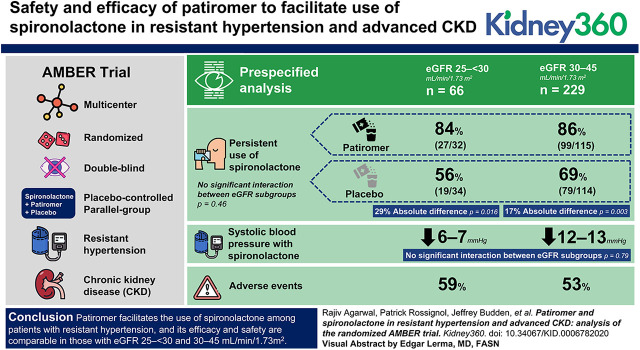
Visual Abstract
Keywords: chronic kidney disease, chronic renal insufficiency, hyperkalemia, patiromer, resistant hypertension, spironolactone
Abstract
Background
Mineralocorticoid receptor antagonists reduce mortality in patients with heart failure with reduced ejection fraction and have become a standard of care in those with resistant hypertension (rHTN). Yet, their use is limited among patients with CKD, primarily due to hyperkalemia.
Methods
AMBER was a multicenter, randomized, double-blind, placebo-controlled, parallel-group study that reported that the use of the potassium-binding drug patiromer allowed a more persistent use of spironolactone in patients with CKD and rHTN. In this report, we compare the safety and efficacy of patiromer in advanced CKD as a prespecified analysis.
Results
Of the 295 patients randomized, 66 fell into the eGFR 25 to <30 subgroup. In this subgroup, persistent use of spironolactone was seen in 19 of 34 (56%) in the placebo group and 27 of 32 (84%) in the patiromer group (absolute difference 29%; P<0.02). In the eGFR 30–45 subgroup, persistent use of spironolactone was seen in 79 of 114 (69%) in the placebo group and 99 of 115 (86%) in the patiromer group (absolute difference 17%; P=0.003). There was no significant interaction between eGFR subgroups (P=0.46). Systolic BP reduction with spironolactone in the eGFR 25 to <30 subgroup was 6–7 mm Hg; in the eGFR 30–45 subgroup, it was 12–13 mm Hg. There was no significant interaction between eGFR subgroups on BP reduction (P=0.79). Similar proportions of patients reported adverse events (59% in the eGFR 25 to <30 subgroup; 53% in the eGFR 30–45 subgroup).
Conclusions
Patiromer facilitates the use of spironolactone among patients with rHTN, and its efficacy and safety are comparable in those with eGFR 25 to <30 and 30–45 ml/min per 1.73 m2.
Clinical Trial registry name and registration number:
Key Points
In the AMBER trial, patiromer enabled more persistent spironolactone use in patients with rHTN and advanced CKD.
Efficacy of patiromer was comparable in prespecified subgroups with eGFR 25 to <30 and 30–45 ml/min per 1.73 m2.
Safety of patiromer was consistent between eGFR subgroups and prior reports, with no new safety signals even in the 25 to <30 eGFR subgroup.
Introduction
The prevalence of apparent resistant hypertension (rHTN) is approximately 10%–15% in the treated hypertensive population (1). The prevalence of rHTN is substantially higher in patients with lower eGFR and higher degrees of albuminuria (2). The Spironolactone versus Placebo, Bisoprolol, and Doxazosin to Determine the Optimal Treatment for Drug rHTN (PATHWAY-2) study demonstrated that among patients with rHTN optimized on a three-drug regimen that included an angiotensin-converting enzyme inhibitor, a calcium channel blocker, and a diuretic, compared with adding an α- or β-blocker, spironolactone elicited a greater BP reduction (3). However, in this trial, patients with eGFR<45 ml/min per 1.73 m2 were excluded, and the average eGFR of the randomized patients was 91 ml/min per 1.73 m2. The United States guidelines recommend adding a mineralocorticoid receptor antagonist (MRA) for patients with rHTN. However, caution is advised for patients with eGFR<30 ml/min per 1.73 m2 (4). The European guidelines recommend adding an MRA for patients with rHTN as long as eGFR is >45 ml/min per 1.73 m2 and plasma potassium (K+) is ≤4.5 mEq/L (5).
In addition to controlling BP, MRA therapy reduces mortality and hospitalization in patients with heart failure (HF) and comorbid CKD (6). The United States HF guidelines recommend MRA use in patients with comorbid HF down to eGFR of 30 ml/min per 1.73 m2 (6). The ESC HF guidelines recommend using MRAs with caution or seeking specialist advice for their use in patients with eGFR<30 ml/min per 1.73 m2 (7), whereas the ACC/AHA/HFSA guidelines note that MRAs may be harmful in patients with eGFR<30 ml/min per 1.73 m2 (6). Finally, a 2017 Kidney Disease Improving Global Outcomes Controversies Conference noted that data were insufficient to recommend the use of MRA to treat rHTN in patients with CKD (8). Notably, this conference was held prior to the publication of AMBER (9). Furthermore, among patients with CKD, compared with placebo, a 2019 meta-analysis showed that the risk for hyperkalemia was increased by approximately 2.6-fold (10). Two consensus statements from the ESC (7,11) suggest the use of novel K+ binders to facilitate MRA use in patients with HF and concomitant CKD. Yet, the utilization of K+ binders in this population remains low. In fact, in patients with HF with reduced ejection fraction, it was noted that only 33% of eligible patients received an MRA in a large registry from the United States (12). In a study from the Americas, Europe, Australia, and Asia encompassing 1555 patients from 76 practices, 95% of the patients with treatment rHTN were prescribed a diuretic, whereas only 36% received MRA (13). The use of MRA in high-risk patients with CKD, diabetes, and HF also remains low (6.6%) (14), and <1% of the patients with hypertension and CKD are prescribed an MRA (15).
The AMBER trial showed that among patients with rHTN and eGFR between 25 and 45 ml/min per 1.73 m2, patiromer, compared with placebo, enabled a more persistent use of spironolactone (9). Patiromer enabled a greater dose of spironolactone and reduced the risk of hyperkalemia by nearly 50%. Here, we report the prespecified subgroup analysis of AMBER primary and secondary end points by eGFR subgroups (eGFR 25 to <30 ml/min per 1.73 m2 [eGFR 25 to <30 subgroup] and 30–45 ml/min per 1.73 m2 [eGFR 30–45 subgroup]).
Materials and Methods
Study Design and Participants
AMBER was a multicenter, randomized, double-blind, placebo-controlled, parallel-group study to evaluate whether patiromer would allow more persistent use of spironolactone in patients with CKD and rHTN. The study design and primary outcomes have previously been published (9,16). AMBER was conducted in accordance with current standards and conforms to the principles outlined in the Declaration of Helsinki, and the study protocol was approved by the institutional review board or independent ethics committee for each institution before study initiation. All patients provided written informed consent before participating in the study.
Eligible patients were age ≥18 years and had an eGFR between 25 and 45 ml/min per 1.73 m2 with serum potassium (sK+) between 4.3 and 5.1 mEq/L. All patients had rHTN during screening, defined as unattended systolic automated office BP (AOBP) of 135–160 mm Hg despite taking three or more antihypertensives, including a diuretic, and an angiotensin-converting enzyme inhibitor or an angiotensin receptor blocker (unless not tolerated or contraindicated). Exclusion criteria included untreated secondary causes of hypertension (other than CKD), recent cardiovascular event (e.g., myocardial infarction, unstable angina, or hospitalization for HF), and clinically significant ventricular arrhythmia or atrial fibrillation with heart rate >100 beats per minute.
AMBER had a screening/run-in period (up to 4 weeks), a double-blind treatment period (12 weeks), and a follow-up visit 2 weeks after the week 12 visit or early termination. The screening period (four visits separated by 4–10 days) ensured that patients were on stable doses of medication, had confirmed treatment rHTN, and met all inclusion criteria. Eligible patients were randomly assigned (1:1) via an interactive web response system at the final screening visit to receive patiromer or matching placebo in addition to open-label spironolactone once daily and their baseline antihypertensive medications, starting on day 1 of randomized treatment. Visits during the double-blind treatment period were weekly (weeks 1–4) and then biweekly (weeks 6–12), at which time AOBP, body weight, blood samples for serum chemistry and spironolactone-level (including metabolites) assessments, and adverse events (AEs) were collected.
At each visit after the initial screening visit, unattended AOBP measurements were recorded for each patient as described (9). Investigators were instructed to keep baseline antihypertensive medications constant except for AE-related reasons, where changes to baseline medications could be justified.
Treatments
Open-label oral spironolactone was started at 25 mg once daily and increased to 50 mg once daily at week 3 in patients with sK+≤5.1 mEq/L if systolic AOBP remained ≥120 mm Hg. Patients initiated study drug (similarly marked packets of patiromer [Relypsa, Inc., a Vifor Pharma Group Company, Redwood City, CA] or placebo) taken once daily with food at least 3 hours before or 3 hours after other medications (including spironolactone). The starting dose of patiromer was 8.4 g once daily. Study drug dosing adjustments were made at intervals of ≥1 week to address hyperkalemia or hypokalemia: upward adjustment to 16.8 g once daily, then 25.2 g once daily for local laboratory sK+ >5.1 mEq/L, and downward adjustment for sK+<4.0 mEq/L. A titration algorithm was also used to reduce or discontinue the dose of spironolactone if decreases in eGFR or hypotension were observed (16).
End Points and Statistical Analyses
This prespecified analysis evaluated AMBER primary and secondary end points by eGFR subgroups (eGFR 25 to <30 subgroup and eGFR 30–45 subgroup). The primary end point was the difference between treatment groups in the proportion of patients remaining on spironolactone at week 12. The secondary efficacy end point was the difference between treatment groups in the change in systolic AOBP from baseline to week 12 (or to the last available measurement before addition of any new antihypertensive medications or increase in any of the baseline antihypertensive medications). Post hoc analyses by baseline eGFR included the following: differences in cumulative spironolactone dose, Kaplan–Meier estimated time to discontinuation of spironolactone, percentage of patients receiving spironolactone 50 mg once daily, daily dose of patiromer, rate of spironolactone discontinuation due to hyperkalemia, Kaplan–Meier estimate of the time to sK+≥5.5 mEq/L, and sK+ over time. Safety was assessed by vital signs, reports of AEs, change in eGFR from baseline, and changes in laboratory parameters (including N-terminal pro B-type natriuretic peptide [NT-proBNP] levels and urine albumin-creatinine ratio). Laboratory assessments included serum calcium (normal range 8.5–10.5 mg/dl) and magnesium (normal range 1.8–2.4 mg/dl) levels over time and the number of patients with prespecified sK+ <3.8 and <3.5 mEq/L, serum calcium >10.5 mg/dl, and serum magnesium <1.4 and <1.2 mg/dl. Efficacy end points and safety were assessed in all randomized patients; all randomized patients received at least one dose of spironolactone and at least one dose of blinded study medication (patiromer or placebo). All laboratory results are on the basis of central laboratory data.
Analysis of the primary end point, between-group differences in the proportion of patients remaining on spironolactone at week 12, used the Cochran–Mantel–Haenszel test stratified by baseline K+ category (4.3 to <4.7 versus 4.7–5.1 mEq/L) and presence/absence of diabetes mellitus. The secondary end point was analyzed using an analysis of covariance model, with baseline systolic AOBP as covariate and the same categorical factors as for the primary end point. Time to discontinuation of spironolactone and time to hyperkalemia (sK+≥5.5 mEq/L) were analyzed using Kaplan–Meier methods, and average daily and cumulative doses of spironolactone were analyzed using analysis of covariance methods. Safety parameters were summarized descriptively. Statistical analyses were performed on SAS software, version 9.4.
Results
Patient Disposition and Baseline Characteristics
In AMBER, 295 patients were randomized to double-blind treatment with either placebo plus spironolactone (n=148) or patiromer plus spironolactone (n=147) in addition to their current treatment regimen of antihypertensive medications. Of these, 66 (22%) patients were in the eGFR 25 to <30 subgroup (34 randomized to placebo and 32 randomized to patiromer), and 229 (78%) patients were in the eGFR 30–45 subgroup (114 randomized to placebo and 115 randomized to patiromer).
In the eGFR 25 to <30 subgroup, 32 (94%) patients randomized to placebo and 32 (100%) patients randomized to patiromer completed the study (109 [96%] and 112 [97%] patients in the eGFR 30–45 subgroup, respectively) (Supplemental Figure 1). The most common reason for study drug discontinuation was meeting a protocol-specified withdrawal criterion for high sK+. In the eGFR 25 to <30 subgroup, study drug discontinuation due to hyperkalemia occurred in nine (26%) patients on placebo and two (6%) patients on patiromer; the discontinuation rates were 25 (22%) on placebo and eight (7%) on patiromer in the eGFR 30–45 subgroup. In the eGFR 25 to <30 and eGFR 30–45 subgroups, 5% and 1% discontinued due to protocol-defined symptomatic hypotension, respectively, and 3% and 2% discontinued due to protocol-defined decline in eGFR, respectively (Supplemental Table 1).
Baseline demographics and disease characteristics were generally similar in both eGFR subgroups (Table 1). In the eGFR 25 to <30 and eGFR 30–45 subgroups, 100% and 97.8% were White, respectively; 39% and 52% had diabetes, respectively; 44% and 45% had a history of HF, respectively; and mean baseline sK+ values were 4.78 and 4.70 mEq/L, respectively.
Table 1.
Baseline characteristics in patients in AMBER by baseline eGFR subgroup and treatment
| Characteristic | eGFR 25 to <30 Subgroup | eGFR 30–45 Subgroup | ||||
| Spironolactone + Placebo, n=34 | Spironolactone + Patiromer, n=32 | Treatment Groups Combined, n=66 | Spironolactone + Placebo, n=114 | Spironolactone + Patiromer, n=115 | Treatment Groups Combined, n=229 | |
| Age, mean (SD), yr | 68.9 (13.4) | 65.1 (13.8) | 67.1 (13.6) | 68.4 (10.4) | 68.5 (11.8) | 68.5 (11.1) |
| ≥65, n (%) | 22 (64.7) | 18 (56.3) | 40 (60.6) | 82 (71.9) | 80 (69.6) | 162 (70.7) |
| White race, n (%) | 34 (100) | 32 (100) | 66 (100) | 111 (97.4) | 113 (98.3) | 224 (97.8) |
| Men, n (%) | 16 (47.1) | 19 (59.4) | 35 (53.0) | 61 (53.5) | 57 (49.6) | 118 (51.5) |
| Systolic AOBP, mean (SD), mm Hg | 144.0 (6.8) | 143.4 (6.7) | 143.7 (6.7) | 145.2 (7.1) | 143.3 (6.5) | 144.2 (6.8) |
| sK+, mean (SD), mEq/L | 4.70 (0.40) | 4.86 (0.35) | 4.78 (0.38) | 4.69 (0.37) | 4.70 (0.36) | 4.70 (0.36) |
| History of hyperkalemia, n (%) | 4 (11.8) | 3 (9.4) | 7 (10.6) | 8 (7.0) | 7 (6.1) | 15 (6.6) |
| Diabetes mellitus, n (%) | 12 (35.3) | 14 (43.8) | 26 (39.4) | 60 (52.6) | 59 (51.3) | 119 (52.0) |
| eGFR, mean (SD), ml/min per 1.73 m2 | 26.0 (3.1) | 26.4 (2.8) | 26.2 (3.0) | 39.1 (5.7) | 37.9 (6.0) | 38.5 (5.9) |
| Serum creatinine, median (Q1, Q3), mg/dl | 2.2 (1.9, 2.4) | 2.3 (1.8, 2.6) | 2.2 (1.8, 2.5) | 1.6 (1.4, 1.8) | 1.6 (1.4, 1.9) | 1.6 (1.4, 1.8) |
| 24-h urine albumin-creatinine ratio, median (Q1, Q3), mg/g | 108 (37, 906) | 304 (37, 1308) | 199 (37, 1092) | 72 (14, 314) | 60 (14, 389) | 70 (14, 364) |
| 24-h adjusted urine sodium, median (Q1, Q3), mEq/L per 24 h | 175 (115, 224) | 146 (102, 205) | 160 (105, 222) | 193 (144, 238) | 183 (131, 264) | 187 (138, 249) |
| History of heart failure, n (%) | 15 (44.1) | 14 (43.8) | 29 (43.9) | 54 (47.4) | 49 (42.6) | 103 (45.0) |
| History of atrial fibrillation, n (%) | 5 (14.7) | 3 (9.4) | 8 (12.1) | 12 (10.5) | 8 (7.0) | 20 (8.7) |
| No. of antihypertensive medications, mean (SD) | 3.7 (0.84) | 3.8 (0.91) | 3.7 (0.87) | 3.6 (0.69) | 3.7 (0.88) | 3.6 (0.79) |
| Use of medications for diabetes, n (%) | 9 (26.5) | 13 (40.6) | 22 (33.3) | 59 (51.8) | 56 (48.7) | 115 (50.2) |
AOBP, automated office BP; sK+, serum potassium; Q, quartile.
Efficacy
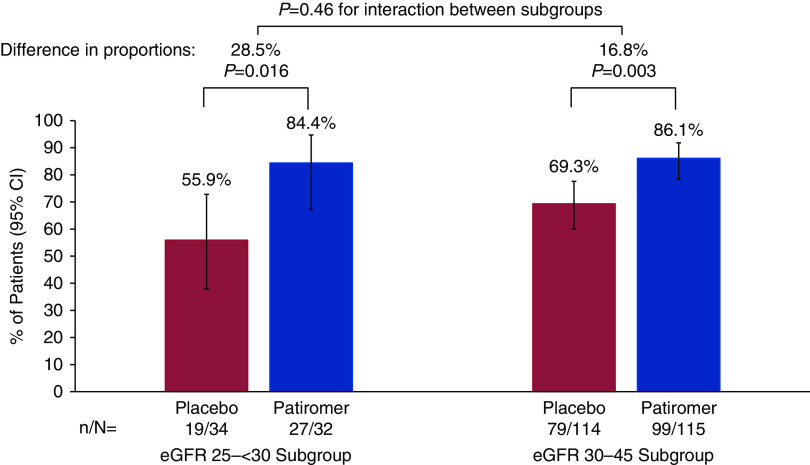
There was no significant interaction between eGFR subgroups (P=0.46) for the primary end point. In the eGFR 25 to <30 subgroup, 55.9% of patients receiving placebo remained on spironolactone at week 12 (Figure 1) compared with 84.4% of patients receiving patiromer (between-group difference =28.5%; 95% confidence interval, 7.6 to 49.4; P<0.02). In the eGFR 30–45 subgroup, 69.3% of patients receiving placebo remained on spironolactone at week 12 compared with 86.1% of patients receiving patiromer (between-group difference =16.8%; 95% confidence interval, 6.2 to 27.4; P=0.003). Kaplan–Meier estimates of the time to early discontinuation of spironolactone are shown in Figure 2. In the eGFR 25 to <30 subgroup, treatment group separation in time to discontinuation of spironolactone began as early as 2 weeks, whereas it was not until 6 weeks that this occurred in the eGFR 30–45 subgroup.
Figure 1.
Percentage of patients who remained on spironolactone at week 12 by eGFR subgroup. 95% CI, 95% confidence interval.
Figure 2.
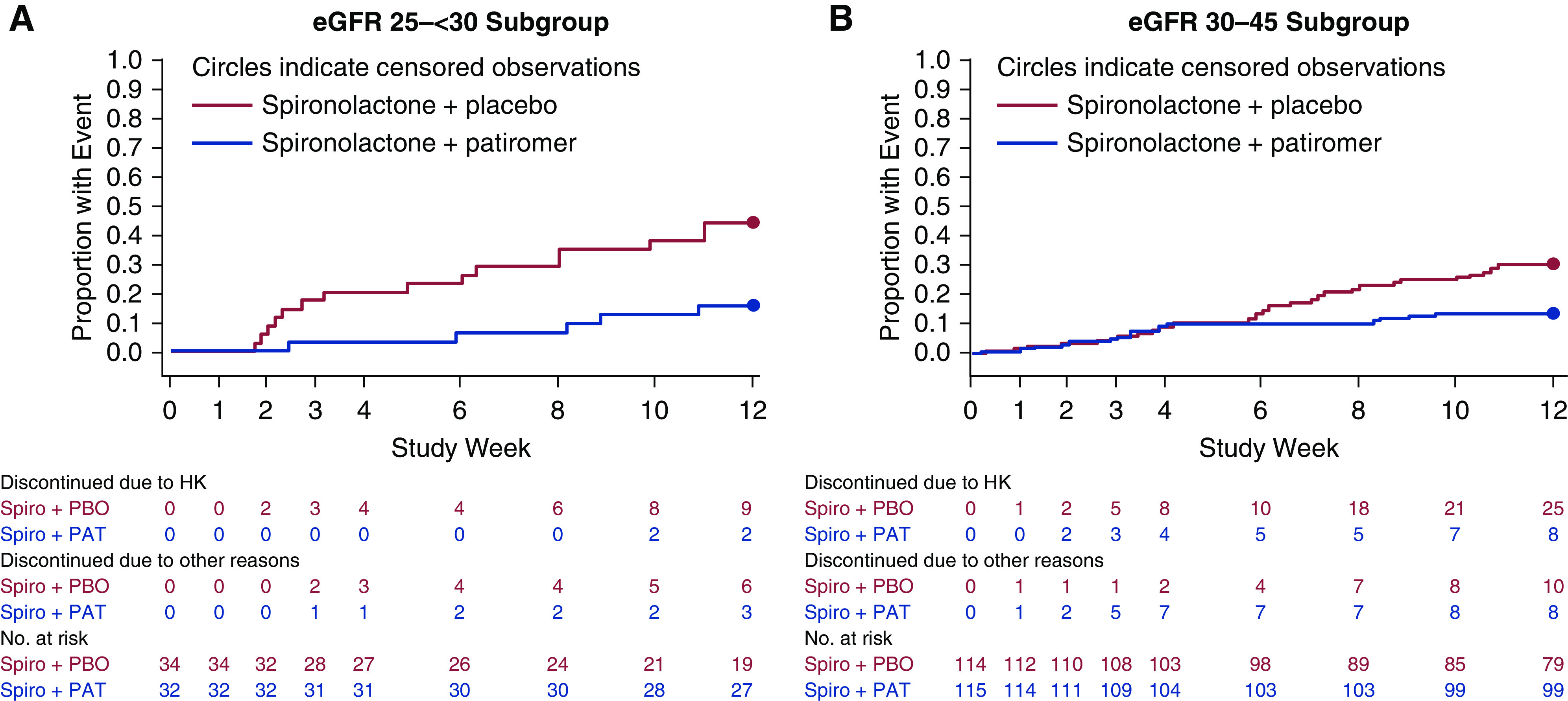
Time to discontinuation of spironolactone (Spiro) in patients. (A) the eGFR 25 to <30 subgroup and (B) the eGFR 30–45 subgroup. Discontinued patients indicate the numbers of patients who discontinued study treatment early for hyperkalemia (HK) or other reasons prior to or at a study visit. PAT, patiromer; PBO, placebo.
The cumulative dose of spironolactone over 12 weeks is shown in Supplemental Table 2. The least squares (LS) mean (SEM) difference between treatment groups (patiromer minus placebo) in cumulative spironolactone dose was 732.4 (274.3) mg in the eGFR 25 to <30 subgroup and 273.8 (139.7) mg in the eGFR 30–45 subgroup. In the eGFR 25 to <30 subgroup, 41% in the placebo group and 72% in the patiromer group were receiving the 50 mg once daily dose of spironolactone at week 12 (54% and 69% in the eGFR 30–45 subgroup, respectively). The LS mean (SEM) differences in average daily dose of spironolactone (patiromer minus placebo) were 4.3 (1.9) mg in the eGFR 25 to <30 subgroup and 1.7 (1.1) mg in the eGFR 30–45 subgroup. Median (quartile 1, quartile 3) daily doses of patiromer were 11.2 (8.4, 16.1) g/d and 9.8 (8.4, 15.9) g/d in the eGFR 25 to <30 and 30–45 subgroups, respectively.
In the eGFR 25 to <30 subgroup, sK+≥5.5 mEq/L occurred in 18 (53%) patients receiving placebo and in 11 (34%) patients receiving patiromer. In the eGFR 30–45 subgroup, sK+≥5.5 mEq/L occurred in 77 (68%) patients receiving placebo and in 41 (36%) patients receiving patiromer. The Kaplan–Meier estimates of the time to first sK+ ≥5.5 mEq/L are shown in Figure 3. The separation between the treatment groups in the occurrence of first sK+ ≥5.5 mEq/L occurred earlier in those in the eGFR 25 to <30 subgroup, evident after week 1. In those in the eGFR 30–45 subgroup, separation between treatment groups was evident after week 3. Mean sK+ over time through week 12 by eGFR subgroup is shown in Supplemental Figure 2.
Figure 3.
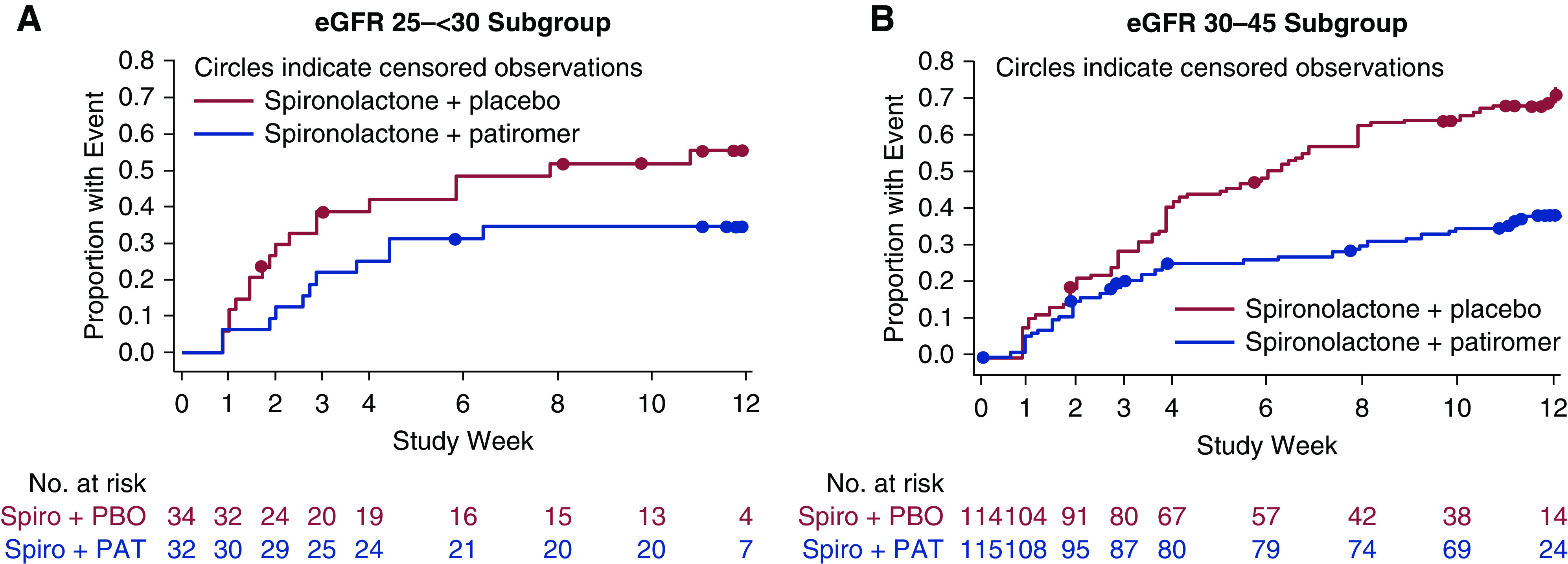
Time to first serum potassium value ≥5.5 mEq/L during treatment in patients. (A) the eGFR 25 to <30 subgroup and (B) the eGFR 30–45 subgroup. The Kaplan–Meier (product-limit) estimates are shown. The number of patients at risk at each time point is the number of patients on treatment and still without event at the end of the time point. The patients who completed 12 weeks of study treatment and had not had any event are censored at week 12. PAT, patiromer; PBO, placebo; Spiro, spironolactone.
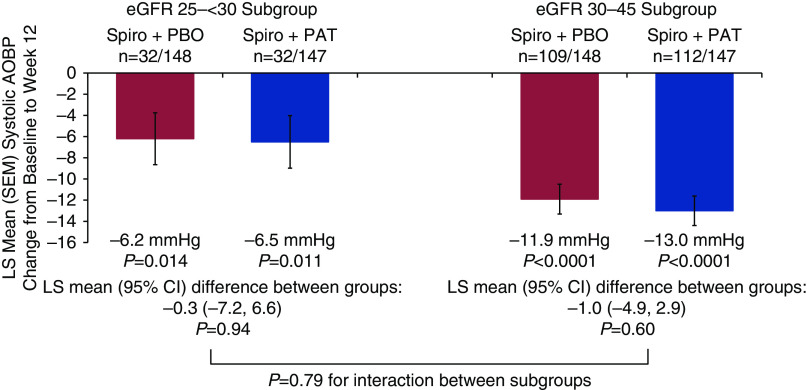
In the eGFR 25 to <30 subgroup, the LS mean (SEM) systolic AOBP reductions from baseline to week 12 are shown in Figure 4 (P<0.02 versus baseline for both treatment groups; P=0.94 for difference between treatment groups). In the eGFR 30–45 subgroup, systolic AOBP reductions were also statistically significant versus baseline for both treatment groups (P<0.001; P=0.60 for difference between treatment groups). There was no significant interaction between eGFR subgroups (P=0.79) for systolic AOBP change from baseline. Additions to antihypertensive medications before week 12 occurred in four patients on placebo (all four in the eGFR 30–45 subgroup) and no patients on patiromer. No additions to antihypertensive medications were reported to be due to new edematous states.
Figure 4.
Least squares (LS) mean (SEM) systolic automated office BP (AOBP) change from baseline to week 12 by eGFR subgroup. 95% CI, 95% confidence interval; PAT, patiromer; PBO, placebo; Spiro, spironolactone.
Safety
Overall similar numbers of patients reported AEs (Table 2): 19 (56%) and 20 (63%) patients randomized to placebo and patiromer, respectively, in the eGFR 25 to <30 subgroup (60 [53%] and 62 [54%] patients, respectively, in the eGFR 30–45 subgroup). AEs were generally mild to moderate in severity. The most frequently occurring class of AEs in both subgroups was gastrointestinal disorders, occurring in five (15%) and nine (28%) patients in the eGFR 25 to <30 subgroup who had been randomized to placebo and patiromer, respectively (in 19 [17%] and 15 [13%], respectively, of those in the eGFR 30–45 subgroup). Diarrhea was the only individual AE within the gastrointestinal class that occurred in four or more patients in the eGFR 25 to <30 subgroup (both treatment groups combined), and it was reported in three (9%) and five (16%) placebo- and patiromer-treated patients, respectively (in five [4.4%] and four [3.5%], respectively, in the eGFR 30–45 subgroup).
Table 2.
Adverse event summary by eGFR subgroups
| Adverse Event | eGFR 25 to <30 Subgroup | eGFR 30–45 Subgroup | ||||
| Spironolactone + Placebo, n=34 | Spironolactone + Patiromer, n=32 | Treatment Groups Combined, n=66 | Spironolactone + Placebo, n=114 | Spironolactone + Patiromer, n=115 | Treatment Groups Combined, n=229 | |
| AEs | 19 (55.9) | 20 (62.5) | 39 (59.1) | 60 (52.6) | 62 (53.9) | 122 (53.3) |
| Severe AEs | 1 (2.9) | 1 (3.1) | 2 (3.0) | 2 (1.8) | 1 (0.9) | 3 (1.3) |
| Serious AEsa | 1 (2.9) | 0 | 1 (1.5) | 3 (2.6) | 1 (0.9) | 4 (1.7) |
| AE leading to study treatment discontinuation | 5 (14.7) | 4 (12.5) | 9 (13.6) | 16 (14.0) | 6 (5.2) | 22 (9.6) |
| AE leading to death | 0 | 0 | 0 | 1 (0.9) | 0 | 1 (0.4) |
| Most common AEs b | ||||||
| Diarrhea | 3 (8.8) | 5 (15.6) | 8 (12.1) | 5 (4.4) | 4 (3.5) | 9 (3.9) |
| Renal impairment | 2 (5.9) | 4 (12.5) | 6 (9.1) | 8 (7.0) | 9 (7.8) | 17 (7.4) |
| Headache | 3 (8.8) | 2 (6.3) | 5 (7.6) | 8 (7.0) | 7 (6.1) | 15 (6.6) |
| Hyperkalemia or blood K+ increased | 2 (5.9) | 3 (9.4) | 5 (7.6) | 12 (10.5) | 6 (5.2) | 18 (7.9) |
| Hypotension | 1 (2.9) | 3 (9.4) | 4 (6.1) | 5 (4.4) | 6 (5.2) | 11 (4.8) |
Data are n (%) of patients with at least one event; each patient is counted only once for each AE. Serum magnesium 1.2–1.4 mg/dl occurred in one patient on placebo and three patients on patiromer in the eGFR 30–45 subgroup; no patients had serum magnesium <1.2 mg/dl. AE, adverse event; K+, potassium.
None were considered related to study drug in the opinion of the investigator.
AEs occurring in four or more patients in the combined treatment groups for the eGFR 25 to <30 subgroup.
Incidence of baseline or any postbaseline sK+ measurement <3.8 mEq/L through week 12 is reported by eGFR subgroup in Supplemental Table 3; one patient in the eGFR 30–45 subgroup receiving patiromer had a postbaseline sK+ measurement between 3.0 and <3.5 mEq/L through week 12. In the eGFR 25 to <30 subgroup, none had sK+<3.5 mEq/L. No patients in either subgroup had sK+<3.0 mEq/L.
In the eGFR 25 to <30 subgroup, one serious AE occurred in a patient receiving placebo (hypersensitivity; considered by the investigator to be unrelated to spironolactone or placebo). In the eGFR 30–45 subgroup, one serious AE occurred in each of four patients: three receiving placebo (renal failure, renal colic, and aortic rupture [the AE leading to death]) and one receiving patiromer (humerus fracture).
Mean changes in eGFR and urine albumin-creatinine ratio are shown in Supplemental Table 4. The changes from baseline were generally similar by eGFR subgroup. AEs indicative of worsening renal function are described in Supplemental Appendix A.
Mean serum calcium and magnesium levels in both eGFR subgroups remained within the normal range in both treatment groups during the study (Supplemental Appendix B, Supplemental Table 5), and the results were not modified by eGFR subgroup.
Discussion
Spironolactone reduces morbidity and mortality in patients with HF with reduced ejection fraction (6,7,17) and is a standard of care among patients with rHTN (3,5,18). However, the use of spironolactone is low in those with congestive HF (12) and patients with treatment rHTN (13) with CKD and/or diabetes (14), and it is rarely prescribed to patients with CKD and hypertension (15). In part, low spironolactone utilization may be because of fear of provoking hyperkalemia. Data from the Swedish Heart Failure Registry have indicated that renin-angiotensin-aldosterone system inhibitors are beneficial in reducing mortality even in patients with stage 4 CKD (19). However, after hyperkalemia occurs, clinical experience shows that renin-angiotensin-aldosterone system inhibitors are promptly discontinued, potentially removing any protective effect of these drugs (20). The AMBER study demonstrated that the use of the K+-binding drug patiromer enables the persistent use of spironolactone in patients with CKD and rHTN (9).
In this analysis, results were similar across CKD subgroups with respect to efficacy in patients with rHTN. However, in the eGFR 25 to <30 subgroup, separation between treatment group curves for discontinuation of spironolactone began as early as 2 weeks, whereas it was not until 6 weeks that this occurred in the eGFR 30–45 subgroup. Spironolactone, regardless of use of placebo or patiromer, provoked a reduction from baseline in systolic BP that was statistically significant. The reduction from baseline in the eGFR 30–45 subgroup was between 12 and 13 mm Hg, whereas in the eGFR 25 to <30 subgroup, it was about 6 mm Hg. There was no evidence of an interaction between baseline eGFR subgroups for either the primary or secondary end point. Patiromer’s safety profile was generally consistent between CKD subgroups and with previous reports (21,22), and this analysis of the AMBER data shows no obvious signal of harm even among patients with eGFR<30 ml/min per 1.73 m2.
As patiromer exchanges K+ for calcium, this may be an important consideration in patients with advanced CKD. Prior studies in healthy adults have suggested that only a small fraction of the calcium released from patiromer is available for absorption (73 mg/d at the highest approved doses of patiromer [25.2 g]) (23). Some of the released calcium from patiromer also may bind to phosphate and thus, reduce serum phosphate levels as demonstrated in prior studies (23,24). In patients with advanced CKD, physicians should consider the risks of a potential small increase in calcium absorption compared with the potential benefits of continuing spironolactone.
Inclusion of patients with CKD and eGFR 25 to <30 is a strength of our study as patients with stage 4 CKD are systematically excluded from randomized, controlled trials (25), and in our study, the retention rate of these patients in a multicenter trial was very high. Our analysis shows that even in the eGFR 25 to <30 subgroup, spironolactone can reduce systolic AOBP by 6 mm Hg, similar to what has been observed by renal denervation trials in ovine models of CKD (26). Inclusion of patients with eGFR 25 to <30 with rHTN is uncommon, and our data demonstrating the safety and efficacy of patiromer with spironolactone are important in this group of patients.
Limitations of AMBER are as follows. Power calculations were performed for the primary analysis as most trials do not have the power to detect interaction effects for subgroups; this is also true for the AMBER trial. The number of patients with stage 4 CKD in this study is small, and therefore, we can only appropriately comment on large differences in safety and efficacy on the basis of CKD stage. Because we found none, considering that this is prespecified subgroup analysis in the statistical analysis plan we can conclude that to the best of our ability the safety and efficacy of patiromer are comparable for early stage 4 CKD and late stage 3 CKD. Enabling spironolactone use with patiromer requires considerations related to managing drug-drug interactions and adds on another therapy to patients who are likely already on multiple medications. On the other hand, enabling steroidal MRA use with patiromer may improve heart and kidney risk, but that will need evaluation in separate studies, such as DIAMOND for cardiovascular outcomes (NCT03888066) and potentially, other studies for kidney failure outcomes. The nonsteroidal MRA finerenone has not been tested in rHTN, and its BP-lowering potential is minimal.
In conclusion, our analyses from AMBER show that patiromer enables the use of spironolactone independent of eGFR within the range studied in AMBER, and the safety and efficacy of patiromer are comparable in patients within this range of advanced CKD.
Disclosures
R. Agarwal reports personal fees from Akebia, AstraZeneca, Bayer, Boehringer Ingelheim, Diamedica, Eli Lilly, Johnson & Johnson, Merck, Reata, Relypsa, Inc., a Vifor Pharma Group Company, and Sanofi; has served as an associate editor of the American Journal of Nephrology and Nephrology Dialysis and Transplantation; is an author on UpToDate; and received research grants from the National Institutes of Health and the US Veterans Administration. S. Arthur reports employment by Relypsa, Inc., a Vifor Pharma Group Company, and stock in Vifor Pharma. J. Budden reports employment by Relypsa, Inc., a Vifor Pharma Group Company, and stock in Vifor Pharma. M. R. Mayo reports previous employment by Relypsa, Inc., a Vifor Pharma Group Company, during the time of the study. P. Rossignol reports consulting for G3P and Idorsia; received honoraria from Ablative Solutions, AstraZeneca, Bayer, Boehringer-Ingelheim, Corvidia, CVRx, Fresenius, Grunenthal, Novartis, NovoNordisk, Relypsa, Inc., a Vifor Pharma Group Company, Sequana Medical, Servier, Stealth Peptides, and Vifor Fresenius Medical Care Renal Pharma; received travel grants from AstraZeneca, Bayer, CVRx, Novartis, and Vifor Fresenius Medical Care Renal Pharma; and is a cofounder of CardioRenal. B. Williams reports honoraria for lectures on hypertension from Boehringer Ingelheim, Daichii Sankyo, Novartis, Pfizer, and Servier and consulting for Novartis, Relypsa, Inc., a Vifor Pharma Group Company, and Vascular Dynamics Inc. W. B. White has nothing to disclose.
Funding
This study was sponsored and funded by Relypsa, Inc., a Vifor Pharma Group Company.
Supplemental Material
This article contains the following supplemental material online at http://kidney360.asnjournals.org/lookup/suppl/doi:10.34067/KID.0006782020/-/DCSupplemental.
(A) Adverse events indicative of worsening renal function and (B) serum calcium and magnesium levels above or below prespecified thresholds during the study. Download Supplemental Appendix, PDF file, 150 KB (149.8KB, pdf)
Reasons for early discontinuation of study treatment by eGFR subgroup and treatment. Download Supplemental Table 1, PDF file, 150 KB (149.8KB, pdf)
Cumulative spironolactone dose over 12 weeks by eGFR subgroup and treatment. Download Supplemental Table 2, PDF file, 150 KB (149.8KB, pdf)
Prespecified laboratory values of interest by eGFR subgroup and treatment. Download Supplemental Table 3, PDF file, 150 KB (149.8KB, pdf)
eGFR and urine albumin-creatinine ratio change from baseline at week 12. Download Supplemental Table 4, PDF file, 150 KB (149.8KB, pdf)
Serum calcium and magnesium levels results over time and change from baseline. Download Supplemental Table 5, PDF file, 150 KB (149.8KB, pdf)
Patient disposition by eGFR subgroups. Download Supplemental Figure 1, PDF file, 150 KB (149.8KB, pdf)
Mean (SEM) central laboratory serum potassium during active treatment in (A) the eGFR 25 to <30 subgroup and (B) the eGFR 30–45 subgroup. Download Supplemental Figure 2, PDF file, 150 KB (149.8KB, pdf)
Acknowledgments
Writing and editorial support services were provided by Impact Communication Partners, Inc. and funded by Relypsa, Inc., a Vifor Pharma Group Company.
A summary of these results was presented at the American Society of Nephrology Kidney Week 2019 (Agarwal R, Rossignol P, Garza D, Mayo MR, Warren S, Ma J, Conrad A, White WB, Williams B: Patiromer versus placebo to enable spironolactone in patients with resistant hypertension and CKD according to baseline kidney function [AMBER Trial]. JASN 30: 14, 2019).
The authors had full access to the data, which were analyzed by the sponsor. All authors were responsible for the interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Author Contributions
R. Agarwal, S. Arthur, M.R. Mayo, P. Rossignol, W.B. White, and B. Williams conceptualized the study; R. Agarwal, P. Rossignol, W.B. White, and B. Williams were responsible for investigation; R. Agarwal, S. Arthur, M.R. Mayo, P. Rossignol, W.B. White, and B. Williams were responsible for methodology; R. Agarwal, S. Arthur, M.R. Mayo, P. Rossignol, W.B. White, and B. Williams provided supervision; R. Agarwal, S. Arthur, J. Budden, M.R. Mayo, P. Rossignol, W.B. White, and B. Williams were responsible for visualization; M.R. Mayo was responsible for project administration and resources; S. Arthur was responsible for data curation, software, validation, and formal analysis; J. Budden and M.R. Mayo were responsible for funding acquisition; and R. Agarwal, S. Arthur, J. Budden, M.R. Mayo, P. Rossignol, W.B. White, and B. Williams reviewed and edited the manuscript.
Data Availability
Individual patient data will be shared. A research proposal must be approved by an independent review panel and the study sponsor, and researchers must sign a data-sharing agreement. Anonymized individual patient-level data will be provided in a secure access environment upon approval of a research proposal and a signed data-sharing agreement. Data can be requested 12 months after the primary publication. Data will be available for a period of 2 years for requests. Proposals for access should be sent to datasharing@viforpharma.com. The AMBER (NCT03071263) study protocol and overall results are posted to the National Institutes of Health clinical trials website (https://clinicaltrials.gov/ct2/show/NCT03071263).
References
- 1.Noubiap JJ, Nansseu JR, Nyaga UF, Sime PS, Francis I, Bigna JJ: Global prevalence of resistant hypertension: A meta-analysis of data from 3.2 million patients. Heart 105: 98–105, 2019. 10.1136/heartjnl-2018-313599 [DOI] [PubMed] [Google Scholar]
- 2.Tanner RM, Calhoun DA, Bell EK, Bowling CB, Gutiérrez OM, Irvin MR, Lackland DT, Oparil S, Warnock D, Muntner P: Prevalence of apparent treatment-resistant hypertension among individuals with CKD. Clin J Am Soc Nephrol 8: 1583–1590, 2013. 10.2215/CJN.00550113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Williams B, MacDonald TM, Morant S, Webb DJ, Sever P, McInnes G, Ford I, Cruickshank JK, Caulfield MJ, Salsbury J, Mackenzie I, Padmanabhan S, Brown MJ; British Hypertension Society’s PATHWAY Studies Group: Spironolactone versus placebo, bisoprolol, and doxazosin to determine the optimal treatment for drug-resistant hypertension (PATHWAY-2): A randomised, double-blind, crossover trial. Lancet 386: 2059–2068, 2015. 10.1016/S0140-6736(15)00257-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Whelton PK, Carey RM, Aronow WS, Casey DE Jr, Collins KJ, Dennison-Himmelfarb C, De Palma SM, Gidding S, Jamerson KA, Jones DW, MacLaughlin EJ, Muntner P, Ovbiagele B, Smith SC Jr, Spencer CC, Stafford RS, Taler SJ, Thomas RJ, Williams KA Sr, Williamson JD, Wright JT Jr: 2017. ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: Executive summary: A report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines [published correction appears in Hypertension 72: e33, 2018 10.1161/HYP.0000000000000080]. Hypertension 71: 1269–1324, 2018 [DOI] [PubMed]
- 5.Williams B, Mancia G, Spiering W, Agabiti Rosei E, Azizi M, Burnier M, Clement DL, Coca A, de Simone G, Dominiczak A, Kahan T, Mahfoud F, Redon J, Ruilope L, Zanchetti A, Kerins M, Kjeldsen SE, Kreutz R, Laurent S, Lip GYH, McManus R, Narkiewicz K, Ruschitzka F, Schmieder RE, Shlyakhto E, Tsioufis C, Aboyans V, Desormais I; ESC Scientific Document Group : 2018 ESC/ESH guidelines for the management of arterial hypertension [published correction appears in Eur Heart J 40: 475, 2019 10.1093/eurheartj/ehy686]. Eur Heart J 39: 3021–3104, 2018. Available at: 10.1093/eurheartj/ehy339 [DOI] [PubMed] [Google Scholar]
- 6.Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr, Colvin MM, Drazner MH, Filippatos GS, Fonarow GC, Givertz MM, Hollenberg SM, Lindenfeld J, Masoudi FA, McBride PE, Peterson PN, Stevenson LW, Westlake C: 2017 ACC/AHA/HFSA focused update of the 2013 ACCF/AHA guideline for the management of heart failure: A report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines and the Heart Failure Society of America. Circulation 136: e137–e161, 2017. 10.1161/CIR.0000000000000509 [DOI] [PubMed] [Google Scholar]
- 7.Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JG, Coats AJ, Falk V, González-Juanatey JR, Harjola VP, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GM, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P; Authors/Task Force MembersDocument Reviewers: 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail 18: 891–975, 2016. 10.1002/ejhf.592 [DOI] [PubMed] [Google Scholar]
- 8.Cheung AK, Chang TI, Cushman WC, Furth SL, Ix JH, Pecoits-Filho R, Perkovic V, Sarnak MJ, Tobe SW, Tomson CRV, Cheung M, Wheeler DC, Winkelmayer WC, Mann JFE; Conference Participants: Blood pressure in chronic kidney disease: Conclusions from a Kidney Disease: Improving Global Outcomes (KDIGO) controversies conference. Kidney Int 95: 1027–1036, 2019. 10.1016/j.kint.2018.12.025 [DOI] [PubMed] [Google Scholar]
- 9.Agarwal R, Rossignol P, Romero A, Garza D, Mayo MR, Warren S, Ma J, White WB, Williams B: Patiromer versus placebo to enable spironolactone use in patients with resistant hypertension and chronic kidney disease (AMBER): A phase 2, randomised, double-blind, placebo-controlled trial. Lancet 394: 1540–1550, 2019. 10.1016/S0140-6736(19)32135-X [DOI] [PubMed] [Google Scholar]
- 10.Alexandrou ME, Papagianni A, Tsapas A, Loutradis C, Boutou A, Piperidou A, Papadopoulou D, Ruilope L, Bakris G, Sarafidis P: Effects of mineralocorticoid receptor antagonists in proteinuric kidney disease: A systematic review and meta-analysis of randomized controlled trials. J Hypertens 37: 2307–2324, 2019. 10.1097/HJH.0000000000002187 [DOI] [PubMed] [Google Scholar]
- 11.Seferovic PM, Ponikowski P, Anker SD, Bauersachs J, Chioncel O, Cleland JGF, de Boer RA, Drexel H, Ben Gal T, Hill L, Jaarsma T, Jankowska EA, Anker MS, Lainscak M, Lewis BS, McDonagh T, Metra M, Milicic D, Mullens W, Piepoli MF, Rosano G, Ruschitzka F, Volterrani M, Voors AA, Filippatos G, Coats AJS: Clinical practice update on heart failure 2019: Pharmacotherapy, procedures, devices and patient management. An expert consensus meeting report of the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail 21: 1169–1186, 2019. 10.1002/ejhf.1531 [DOI] [PubMed] [Google Scholar]
- 12.Greene SJ, Butler J, Albert NM, DeVore AD, Sharma PP, Duffy CI, Hill CL, McCague K, Mi X, Patterson JH, Spertus JA, Thomas L, Williams FB, Hernandez AF, Fonarow GC: Medical therapy for heart failure with reduced ejection fraction: The CHAMP-HF Registry. J Am Coll Cardiol 72: 351–366, 2018. 10.1016/j.jacc.2018.04.070 [DOI] [PubMed] [Google Scholar]
- 13.Carcel C, Neal B, Oparil S, Rogers K, Narkiewicz K, Wang JG, Schiffrin EL, Poulter N, Azizi M, Chalmers J: Clinical characteristics, antihypertensive medication use and blood pressure control among patients with treatment-resistant hypertension: The survey of PatIents with treatment ResIstant hyperTension study. J Hypertens 37: 2216–2224, 2019. 10.1097/HJH.0000000000002184 [DOI] [PubMed] [Google Scholar]
- 14.Blankenburg M, Fett AK, Eisenring S, Haas G, Gay A: Patient characteristics and initiation of mineralocorticoid receptor antagonists in patients with chronic kidney disease in routine clinical practice in the US: A retrospective cohort study. BMC Nephrol 20: 171, 2019. 10.1186/s12882-019-1348-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alencar de Pinho N, Levin A, Fukagawa M, Hoy WE, Pecoits-Filho R, Reichel H, Robinson B, Kitiyakara C, Wang J, Eckardt KU, Jha V, Oh KH, Sola L, Eder S, de Borst M, Taal M, Feldman HI, Stengel B; International Network of Chronic Kidney Disease cohort studies (iNET-CKD): Considerable international variation exists in blood pressure control and antihypertensive prescription patterns in chronic kidney disease. Kidney Int 96: 983–994, 2019. 10.1016/j.kint.2019.04.032 [DOI] [PubMed] [Google Scholar]
- 16.Agarwal R, Rossignol P, Garza D, Mayo MR, Warren S, Arthur S, Romero A, White WB, Williams B: Patiromer to enable spironolactone use in the treatment of patients with resistant hypertension and chronic kidney disease: Rationale and design of the AMBER Study. Am J Nephrol 48: 172–180, 2018. 10.1159/000492622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pitt B, Zannad F, Remme WJ, Cody R, Castaigne A, Perez A, Palensky J, Wittes J; Randomized Aldactone Evaluation Study Investigators: The effect of spironolactone on morbidity and mortality in patients with severe heart failure. N Engl J Med 341: 709–717, 1999. 10.1056/NEJM199909023411001 [DOI] [PubMed] [Google Scholar]
- 18.Carey RM, Calhoun DA, Bakris GL, Brook RD, Daugherty SL, Dennison-Himmelfarb CR, Egan BM, Flack JM, Gidding SS, Judd E, Lackland DT, Laffer CL, Newton-Cheh C, Smith SM, Taler SJ, Textor SC, Turan TN, White WB; American Heart Association Professional/Public Education and Publications Committee of the Council on Hypertension; Council on Cardiovascular and Stroke Nursing; Council on Clinical Cardiology; Council on Genomic and Precision Medicine; Council on Peripheral Vascular Disease; Council on Quality of Care and Outcomes Research; and Stroke Council: Resistant hypertension: Detection, evaluation, and management: A scientific statement from the American Heart Association. Hypertension 72: e53–e90, 2018. 10.1161/HYP.0000000000000084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Edner M, Benson L, Dahlström U, Lund LH: Association between renin-angiotensin system antagonist use and mortality in heart failure with severe renal insufficiency: A prospective propensity score-matched cohort study. Eur Heart J 36: 2318–2326, 2015. 10.1093/eurheartj/ehv268 [DOI] [PubMed] [Google Scholar]
- 20.Epstein M, Reaven NL, Funk SE, McGaughey KJ, Oestreicher N, Knispel J: Evaluation of the treatment gap between clinical guidelines and the utilization of renin-angiotensin-aldosterone system inhibitors. Am J Manag Care 21[Suppl]: S212–S220, 2015 [PubMed] [Google Scholar]
- 21.Bakris GL, Pitt B, Weir MR, Freeman MW, Mayo MR, Garza D, Stasiv Y, Zawadzki R, Berman L, Bushinsky DA; AMETHYST-DN Investigators : Effect of patiromer on serum potassium level in patients with hyperkalemia and diabetic kidney disease: The AMETHYST-DN Randomized Clinical Trial [published correction appears in JAMA 314: 731, 2015 10.1001/jama.2015.9237]. JAMA 314: 151–161, 2015. Available at: 10.1001/jama.2015.7446 [DOI] [PubMed] [Google Scholar]
- 22.Weir MR, Bakris GL, Bushinsky DA, Mayo MR, Garza D, Stasiv Y, Wittes J, Christ-Schmidt H, Berman L, Pitt B; OPAL-HK Investigators: Patiromer in patients with kidney disease and hyperkalemia receiving RAAS inhibitors. N Engl J Med 372: 211–221, 2015. 10.1056/NEJMoa1410853 [DOI] [PubMed] [Google Scholar]
- 23.Bushinsky DA, Spiegel DM, Gross C, Benton WW, Fogli J, Hill Gallant KM, Du Mond C, Block GA, Weir MR, Pitt B: Effect of patiromer on urinary ion excretion in healthy adults. Clin J Am Soc Nephrol 11: 1769–1776, 2016. 10.2215/CJN.01170216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bushinsky DA, Spiegel DM, Yuan J, Warren S, Fogli J, Pergola PE: Effects of the potassium-binding polymer patiromer on markers of mineral metabolism. Clin J Am Soc Nephrol 14: 103–110, 2019. 10.2215/CJN.04500418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rossignol P, Massy ZA, Azizi M, Bakris G, Ritz E, Covic A, Goldsmith D, Heine GH, Jager KJ, Kanbay M, Mallamaci F, Ortiz A, Vanholder R, Wiecek A, Zoccali C, London GM, Stengel B, Fouque D; ERA-EDTA EURECA-m working group: Red de Investigación Renal (REDINREN) network; Cardiovascular and Renal Clinical Trialists (F-CRIN INI-CRCT) network : The double challenge of resistant hypertension and chronic kidney disease. Lancet 386: 1588–1598, 2015. Available at: 10.1016/S0140-6736(15)00418-3 [DOI] [PubMed] [Google Scholar]
- 26.Singh RR, Denton KM: Renal denervation. Hypertension 72: 528–536, 2018. 10.1161/HYPERTENSIONAHA.118.10265 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(A) Adverse events indicative of worsening renal function and (B) serum calcium and magnesium levels above or below prespecified thresholds during the study. Download Supplemental Appendix, PDF file, 150 KB (149.8KB, pdf)
Reasons for early discontinuation of study treatment by eGFR subgroup and treatment. Download Supplemental Table 1, PDF file, 150 KB (149.8KB, pdf)
Cumulative spironolactone dose over 12 weeks by eGFR subgroup and treatment. Download Supplemental Table 2, PDF file, 150 KB (149.8KB, pdf)
Prespecified laboratory values of interest by eGFR subgroup and treatment. Download Supplemental Table 3, PDF file, 150 KB (149.8KB, pdf)
eGFR and urine albumin-creatinine ratio change from baseline at week 12. Download Supplemental Table 4, PDF file, 150 KB (149.8KB, pdf)
Serum calcium and magnesium levels results over time and change from baseline. Download Supplemental Table 5, PDF file, 150 KB (149.8KB, pdf)
Patient disposition by eGFR subgroups. Download Supplemental Figure 1, PDF file, 150 KB (149.8KB, pdf)
Mean (SEM) central laboratory serum potassium during active treatment in (A) the eGFR 25 to <30 subgroup and (B) the eGFR 30–45 subgroup. Download Supplemental Figure 2, PDF file, 150 KB (149.8KB, pdf)