Abstract
OBJECTIVE
To describe clinical, diagnostic, and epidemiological features of an outbreak of leptospirosis in dogs in Maricopa County, Ariz, from January 2016 through June 2017.
ANIMALS
71 case and 281 control dogs.
PROCEDURES
Cases were classified as confirmed, probable, suspect, or not a case on the basis of medical record data that fulfilled clinical, diagnostic, and epidemiological criteria. Potential exposures were assessed by owner survey. For the case-control investigation, control dogs were recruited through owner completion of a July 2017 survey. Summary statistics and ORs for case dog lifestyle factors were reported.
RESULTS
54 dogs were classified as confirmed and 17 as probable cases. For 4 dogs of a household cluster (5 confirmed and 3 probable), the highest microscopic agglutination titer was for serovar Djasiman (Leptospira kirschneri detected by PCR assay), and for 13 dogs of a community outbreak (49 confirmed and 14 probable cases), the highest titer was for serovar Canicola (Leptospira interrogans detected by PCR assay). The 44 case dogs included in the case-control investigation were 7.7 (95% CI, 3.5 to 16.7) and 2.9 times (95% CI, 1.3 to 6.6) as likely as control dogs to have visited dog daycare or to have been kenneled overnight at a boarding facility, respectively, 30 days prior to the onset of clinical signs or diagnosis.
CONCLUSIONS AND CLINICAL RELEVANCE
Diagnostic and epidemiological findings indicated 2 outbreaks. Transmission where dogs congregated likely propagated the community outbreak. Outbreaks of leptospiral infections can occur in regions of low prevalence, and a dog’s exposure to areas where dogs congregate should be considered when making Leptospira vaccination recommendations.
Leptospirosis is caused by spirochete bacteria of the genus Leptospira, which can infect many mammals, with species Leptospira kirschneri and Leptospira interrogans the most common causes of disease in dogs.1 Leptospires are further classified into antigenically related serogroups composed of serovars, with L interrogans serovars Autumnalis, Bratislava, Canicola (dogs are the reservoir host), Icterohemorrhagiae, and Pomona and L kirschneri serovar Grippotyphosa thought to be the primary causes of disease in dogs in the United States.1,2 Leptospires are maintained in the renal tubules and are shed in the urine of mammalian reservoir hosts. Transmission occurs when an animal comes in contact with urine or an environment (water, soil, or food) contaminated with urine from a Leptospira-infected animal; leptospires enter the body through mucous membranes, abraded skin, or ingestion.3 Infected dogs can pose a zoonotic disease risk to veterinarians, animal care staff, and dog owners from exposure to the dog’s urine. Because of this risk, Arizona veterinarians and veterinary diagnostic laboratories are required to report suspected or confirmed cases of canine leptospirosis to the state veterinarian (Arizona administrative code R3-2-402).
Infection in dogs can be subclinical3,4 such that infection is rarely detected, can induce mild, nonspecific clinical signs, or can manifest as severe disease, including kidney disease, liver failure, and pulmonary hemorrhagic syndrome. Canine bivalent (serovars Canicola and Icterohemorrhagiae) and quadrivalent (serovars Canicola, Icterohemorrhagiae, Pomona, and Grippotyphosa) vaccines are available in North America, although their use likely varies geographically.1 Diagnostic testing options for leptospirosis include PCR assay of whole blood and urine samples and serologic tests, including ELISA and lateral flow point-of-care tests (screening serologic tests) and the MAT (reference serologic test). Following leptospirosis diagnosis, the preferred treatment is doxycycline at 5 mg/kg (2.3 mg/lb) twice daily for 14 days.1
Maricopa County, Ariz, which includes the cities of Phoenix, Scottsdale, and Tempe, encompasses > 9,000 square miles of arid desert with high temperatures, low annual rainfall, and low relative humidity; these conditions are not thought to favor persistence of Leptospira bacteria in the environment,1,5–8 and reported infections in people and dogs are rare. From 2006 through 2017 in Arizona, 4 probable human cases of leptospirosis were reported; all were associated with travel out of the state or country. From 2011 through 2016, < 5 cases of canine leptospirosis were reported to the state veterinarian.
However, in 2016, 2 clusters of cases of canine leptospirosis were reported in Maricopa County: 9 suspected cases from 1 household in February and 18 suspected cases from 1 boarding facility in November. Two additional suspected clusters at different boarding facilities and sporadic individual cases were reported during January 2017. The unexpected increase in reports of canine leptospirosis in Maricopa County and the concurrent risk for human infections led public and animal health officials to investigate these cases and potential sources of infection. The purposes of the investigation reported here were to characterize the clinical features and diagnostic test results of dogs that resided in Maricopa County and were reported to have leptospirosis and to identify the epidemiological features of infection. Because of the 3 reports of clusters of canine leptospirosis at boarding facilities within 3 months, we hypothesized that dogs that had more regular and frequent contact with other dogs or that were frequently in areas where dogs congregated were at greater odds for infection.
Materials and Methods
Case series
Ascertainment of cases—
Dogs with an onset of illness that was confirmed or suspected to be caused by Leptospira spp between January 1, 2016, and June 30, 2017, whose owners’ primary residence was in Maricopa County were included. Cases were reported by veterinarians, owners of dog daycare and boarding facilities, and dog owners to the state veterinarian or the state or local public health departments. The dog owners and personnel at veterinary clinics and daycare and boarding facilities were contacted to determine whether any people were ill after contact with an infected dog.9 Additionally, a summary of a dog’s clinical course and exposure history (travel, lifestyle, and activities) and a copy of the medical records related to a diagnosis of leptospirosis were requested. Investigation activities were reviewed and determined to be nonresearch by the delegated authority at the CDC’s Center for Surveillance, Epidemiology, and Laboratory Services.
Medical record review—
Medical records were reviewed, and data regarding signalment, clinical signs, physical examination findings, Leptospira vaccination history, treatment, hospitalization, and prescribed antimicrobials were extracted and entered into an electronic database.a Also extracted were results of CBC and serum biochemical analyses at presentation, plus each analyte’s highest or lowest value observed over the course of care, and results of diagnostic tests for the detection of leptospiral DNA and anti-Leptospira antibodies.
Diagnostic testing for leptospirosis—
Diagnostic tests for leptospirosis were selected at the discretion of the attending veterinarian. Testing included rapid point-of-care testsb or laboratory ELISAc (both qualitative) for IgM and IgG antibodies (serum), MAT (serum), and PCR assays (whole blood and urine samples). Microscopic agglutination testing was performed at 5 laboratories (CDC10 and 2 commercial and 2 university veterinary diagnostic laboratories; Supplementary Appendix S1, available at: avmajournals.avma.org/doi/suppl/10.2460/javma.258.6.616). If initial test results were not sufficient to confirm or rule out infection on the basis of the case definition, additional testing was recommended. After leptospiral DNA was detected in blood and urine samples with a PCR assay performed at the CDC,11 samples were subjected to a follow-up Leptospira species-specific PCR assay12 to identify the infecting Leptospira spp (L interrogans, L kirschneri, Leptospira noguchii, or Leptospira borgpetersenii). When possible, Ellinghausen-McCullough-Johnson-Harris semisolid culture media were inoculated with urine samples in an attempt to isolate Leptospira spp; cultures with no leptospiral growth after 6 months were reported as negative.
Case definition—
A case was defined by clinical, laboratory, and epidemiological criteria (Appendix). Briefly, cases were considered as confirmed leptospirosis on the basis of the following diagnostic criteria13–17: Leptospira DNA detected by means of a PCR assay in any sample or an MAT titer of ≥ 1:400 identified for a dog not vaccinated against Leptospira spp or ≥ 1:800 identified for a dog with vaccination ≥ 6 months prior to testing, unknown vaccination status, or known vaccination but unknown date of vaccination. A confirmed diagnosis was also possible for dogs vaccinated < 6 months prior to MAT, but criteria for MAT titers were higher.
A case was classified as probable leptospirosis when a dog met a combination of 2 clinical, supportive diagnostic, or epidemiological criteria. Clinical criteria were met when a dog had ≥ 2 nonspecific clinical signs or physical examination findings or ≥ 1 of several serum biochemical abnormalities or clinical signs likely to be associated with leptospirosis.1,18–20 Dogs that did not meet the clinical criteria but met supportive diagnostic and epidemiological criteria were classified as probable cases only when they had never been vaccinated against Leptospira spp. Detection of IgM and IgG antibodies with the point-of-care test or laboratory ELISA and variable but low MAT titers (vs MAT titers for confirmed cases), depending on a dog’s vaccination status, met the supportive diagnostic criteria. Epidemiological criteria were met for a dog that was exposed to another dog in the household with confirmed leptospirosis, was kenneled or had attended dog daycare at a facility where a dog with confirmed leptospirosis had also been, or had direct contact with a dog with confirmed leptospirosis, as reported by the dog owner.
A case was classified as suspect when a dog met the clinical criteria alone or supportive diagnostic criteria alone and did not meet any other case definition. A dog was classified as not a case when it had no detectable anti-Leptospira antibodies in a blood sample collected ≥ 10 days after the onset of illness. Dogs that lacked sufficient evidence to meet any case definition were excluded from analysis.
Nested case-control investigation
Selection of cases and controls—
All confirmed and probable cases not associated with the January to February 2016 household cluster and for which owner contact information was available were eligible for the case-control investigation. Each owner was contacted by telephone between February 15 and June 20, 2017, and was invited to complete a telephone or web-based surveyd about their dog’s illness, lifestyle, and activities that could have led to Leptospira exposure. Control dogs were recruited from dog owners throughout Maricopa County by means of a different web-based surveyd that also assessed the same lifestyle and activity factors as case dogs. During July 2017, an anonymous link was posted on social media and to a public health internal email list and electronically sent to 7 veterinary clinics or boarding facilities that reported recent cases of canine leptospirosis (from a group of 25 clinics or boarding facilities that reported cases at any time) with a request that they distribute the survey link to clients and staff. Dog owners were asked to complete the survey for only 1 dog/household. To reduce potential misclassification of cases as controls, each owner was asked whether all dogs in their household had been generally healthy in the previous 6 months. Dogs were excluded if their household included any dogs that had previously had leptospirosis or clinical signs consistent with leptospirosis (eg, vomiting, diarrhea, and anorexia) or if owners reported administering antimicrobials to their dogs.
Exposure assessment—
We were most interested in locations where dogs would have substantial contact with other dogs and environments potentially contaminated by Leptospira-infected dogs. We developed a standardized data collection form to record information about case dogs’ exposures in the 30 days (the maximum incubation period in people21) prior to the onset of clinical signs or date of diagnosis for dogs lacking clinical signs; exposures could have occurred in dog daycare, obedience class, and boarding and grooming facilities; at dog parks and shows; and on hiking trails. For control dogs, we asked about the same possible exposures, including the frequency of visits to dog daycare facilities and dog parks, in the previous 30 days and 6 months. On the basis of the frequency of their exposure to dog daycare facilities or dog parks in the previous 30 days, dogs were assigned to 1 of 3 groups as follows: dogs assigned to the high-exposure group had ≥ 1 potential exposure/wk, the moderate-exposure group had ≥ 1 potential exposure/30 d but < 1/wk, and the low-exposure group had no known potential exposures. Other possible exposures related to lifestyle and the home environment were also assessed for case and control dogs, such as travel, contact with standing water, food and water bowl location (indoors vs outdoors), proportion of time spent outdoors, and contact with rodents, wildlife, or livestock.
Data analysis
Data were managed in survey softwared and electronic databases,a and analysis was performed with a statistical software program.e Frequencies and summary statistics (mean and SD for parametric data and median and range for nonparametric data) for clinical and exposure variables were calculated, and values for case dogs were compared with those for control dogs by use of the 2-sided t test or Wilcoxon rank sum test. To facilitate interpretation of results, age groups were created (< 1 year, 1 to 3 years, 4 to 7 years, 8 to 10 years, and > 10 years) and body weight was dichotomized (< 15 kg [33 lb] and ≥ 15 kg) to approximate the sizes of small- and large-breed dogs.
For the case-control analysis, the Pearson χ2 test or 2-sided Fisher exact test (when ≥ 20% of cells of a contingency table had expected counts < 5) was used to explore relationships between dog characteristics and exposure variables among case and control dogs. The OR was calculated for each dog characteristic, exposure, and lifestyle variable with univariable logistic regression models to independently assess the odds associated with each of these variables. An adjusted OR was also calculated for each dog exposure and lifestyle variable in a series of multivariable logistic regression models adjusted for conceptualized confounders identified a priori; for each variable of interest, we adjusted for age (years), body weight (< 15 kg or ≥ 15 kg), sex (male or female), and neuter status (sexually intact or neutered). Multivariable models for exposure and lifestyle variables (eg, boarding, dog daycare, dog parks, hiking, obedience school, dog shows, groomers, and pet stores and contact with rodents, livestock, and wildlife) that could be confounded by a dog owner’s socioeconomic status were also adjusted for the median household income of the owner’s tract as determined by the US Census Bureau.22 We were unable to control for vaccination status in the models because no case dogs had documented receipt of a 2-dose series of Leptospira vaccine prior to exposure. Therefore, the data were reanalyzed (sensitivity analysis) with the exclusion of all control dogs whose owners indicated that their dogs had received a Leptospira vaccine or that they were unsure whether their dogs had ever received a vaccine (ie, case dogs compared with subset of control dogs that had never been vaccinated). Values of P < 0.05 were considered significant.
Results
Case series
Diagnostic testing for leptospirosis—
Eighty-eight suspected cases of canine leptospirosis were reported to the state veterinarian and local or state public health departments from February 2016 through June 2017. Partial or complete medical records were available for 83 cases. Diagnostic testing for leptospirosis was sufficient to classify 60 (68%) dogs as a confirmed case or not a case; 27 (31%) dogs had at least 1 diagnostic test performed, but results were not sufficient to confirm or rule out infection according to the case definition. One dog was not tested. A serologic screening test (point-of-care test or laboratory ELISA) was performed on samples from 45 (51%) dogs, a MAT on samples from 34 (39%) that included 1 dog with results for acute and convalescent samples, and a PCR assay on samples from 66 (75%). Culture media inoculated with 7 urine samples from 5 dogs (2 confirmed cases and 3 probable cases) did not yield leptospiral growth.
Case classification and timing—
Fifty-four (61%) cases were classified as confirmed, 17 (19%) as probable, 5 (6%) as suspect, and 6 (7%) as not a case; 6 (7%) cases were excluded (Figure 1; Supplementary Figure S1, available at: avmajournals.avma.org/doi/suppl/10.2460/javma.258.6.616). No dogs classified as confirmed or probable cases had documentation of vaccination against Leptospira spp (administration of a 2-dose series) prior to the onset of clinical signs. The vaccination history for 8 of 71 dogs that were confirmed or probable cases of leptospirosis was unknown, and 3 dogs vaccinated in response to a boarding facility outbreak were administered 1 dose of Leptospira vaccine 3, 13, and 15 days prior to the onset of clinical signs.
Figure 1—
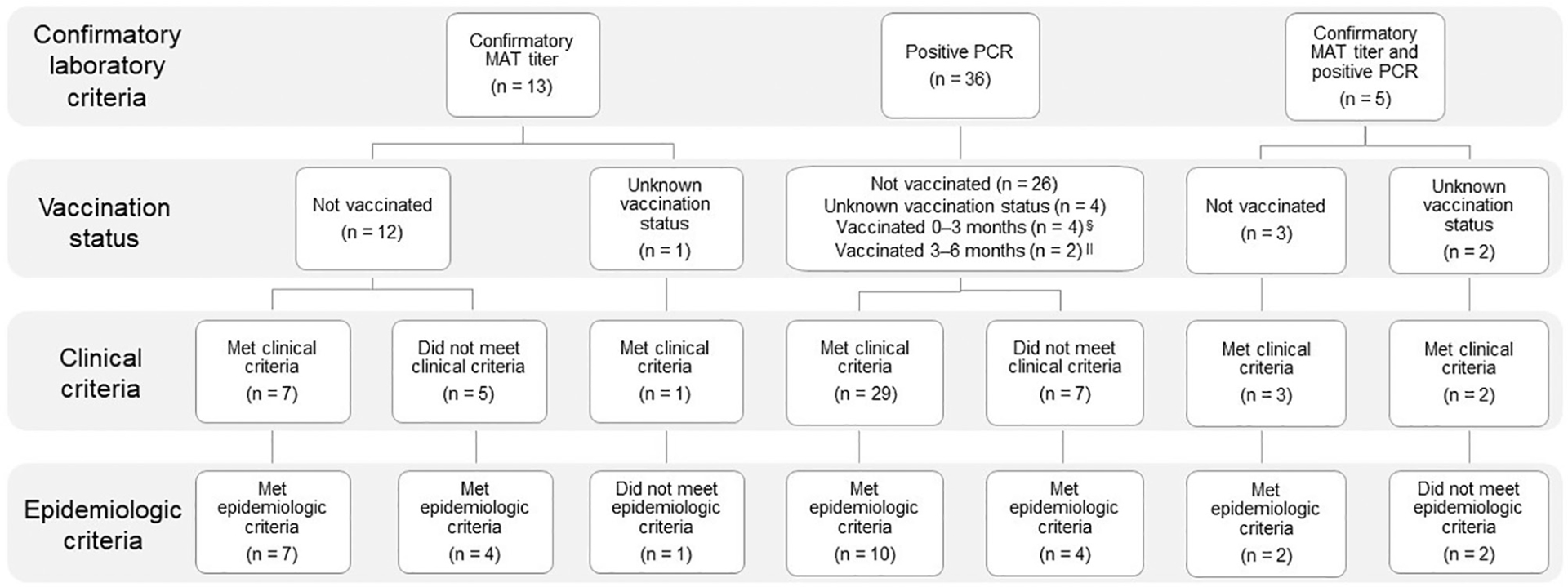
Confirmed* cases (n = 54) of leptospirosis that were reported by veterinarians, owners of dog daycare or boarding facilities, and dog owners to the state veterinarian or state or local public health departments between January 1, 2016, and June 30, 2017, in Maricopa County, Ariz, enumerated by method of confirmation and Leptospira vaccination status,† and whether clinical and epidemiological criteria of the outbreak case definition were met.‡ *Confirmed case = Positive PCR assay result for any biological specimen, MAT titer ≥ 1:400 for unvaccinated dogs or ≥ 1:800 for dogs with unknown vaccination status, or a 4-fold rise in MAT titer for an unvaccinated dog or a dog vaccinated ≥ 2 months prior to sample collection. †Vaccination status relative to diagnostic sample collection. ‡Clinical criteria were met when a dog had ≥ 2 nonspecific clinical signs or physical examination findings (fever [rectal temperature ≥ 39.4°C {103°F}]; lethargy; anorexia; vomiting, diarrhea, or abdominal pain; muscle or joint tenderness; chemosis or conjunctivitis; or dyspnea, tachypnea, or cough) or ≥ 1 serum biochemical abnormality or clinical sign likely to be associated with leptospirosis, as follows: acute kidney injury (high serum creatinine concentration with or without polyuria, polydipsia, oliguria, or anuria), icterus or hyperbilirubinemia, acute increase in liver enzyme activities, uveitis, abortion, or pulmonary hemorrhage or other unexplained bleeding. Epidemiological criteria were met if a dog was exposed to another dog in the household with confirmed leptospirosis, was kenneled or attended daycare at a facility where a dog with confirmed leptospirosis had also been, or had direct contact with a dog with confirmed leptospirosis, as reported by the dog’s owner. §Three dogs received their first dose of Leptospira vaccine 3, 13, and 15 days prior to the onset of clinical signs. ∥Dogs were vaccinated in response to a boarding facility outbreak. One dog did not have clinical signs, and the other dog had clinical signs and was vaccinated 8 days after onset but prior to the collection of samples for diagnostic testing.
For these 71 confirmed and probable cases, date of illness onset or, for dogs lacking clinical signs, date of diagnosis ranged from January 2016 to June 2017, with 2 distinct outbreaks in January to February 2016 and October 2016 to June 2017 (Figure 2). The first outbreak consisted of 8 dogs, including several show dogs, from 1 household; 1 dog traveled to Florida and Southern California for dog shows within 30 days prior to the onset of clinical signs. The dogs’ home was located at the boundary between a suburban and rural area of Maricopa County, and the dog owner reported that wildlife frequently accessed the property. Most (63 [89%]) cases were associated with the second community outbreak (ie, main outbreak).
Figure 2—
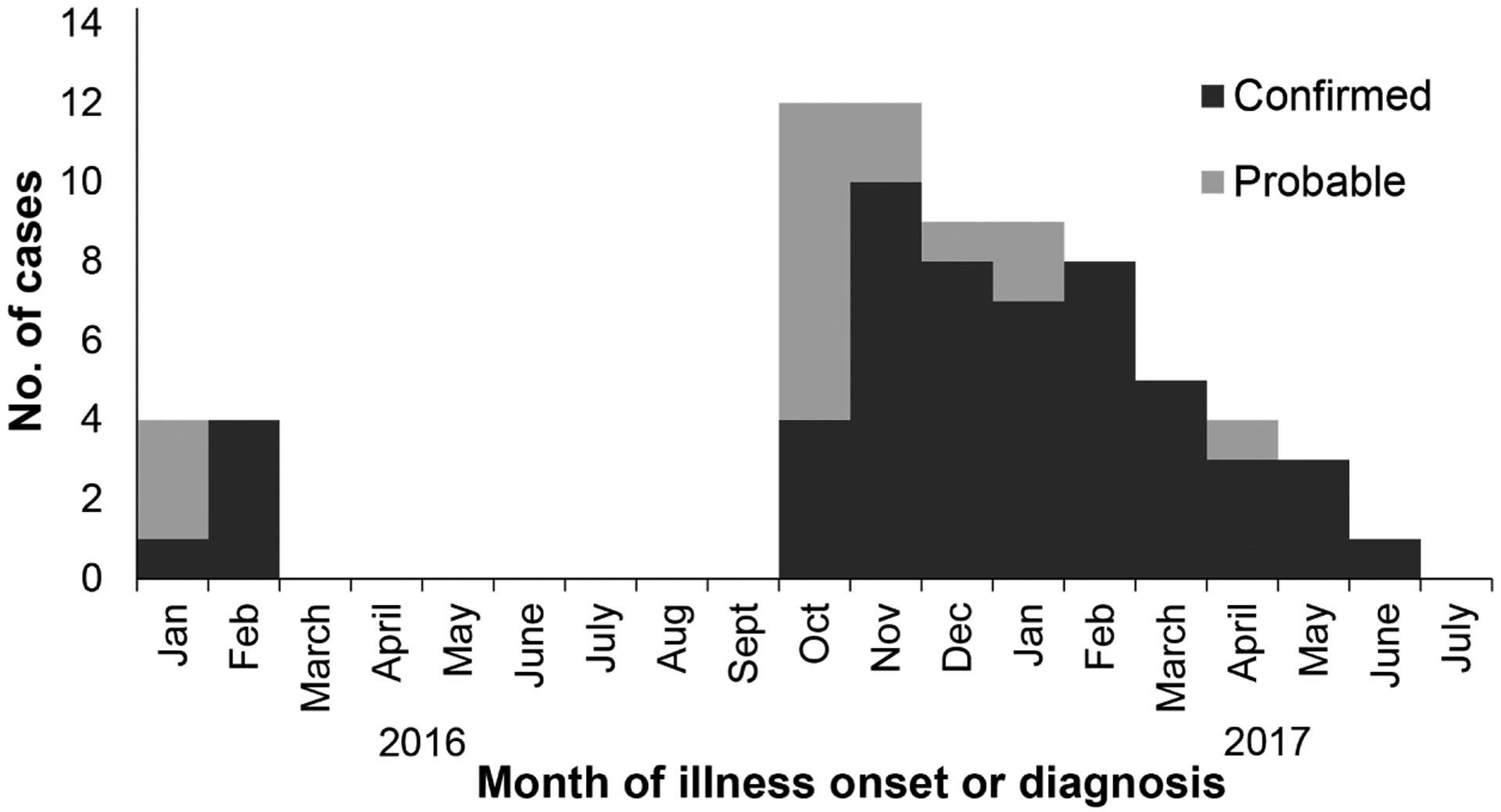
Confirmed (n = 54) and probable (17) cases of leptospirosis by month of illness onset (n = 64) or, for dogs lacking clinical signs, by month of diagnosis (confirmed cases, 6; probable cases, 1) between January 1, 2016, and June 30, 2017, in Maricopa County, Ariz, as reported by veterinarians, owners of dog daycare or boarding facilities, and dog owners to the state veterinarian or state or local public health departments.
Diagnostic testing of confirmed and probable cases—
At least 1 specimen from 52 of 71 (73%) confirmed and probable cases was analyzed with a PCR assay (Table 1); 1 of 2 kidney specimens, 15 of 52 (29%) whole blood specimens, and 33 of 53 (62%) urine specimens had detectable leptospiral DNA (positive result). Positive blood samples were collected earlier (median, 2 days) after illness onset than were negative blood samples (median, 8 days; P < 0.01). No blood samples collected after initiating antimicrobial administration were positive, but 11 urine samples from 11 dogs (11/22 [50%]) collected after initiating antimicrobial administration were positive; 3 samples were from dogs that had received doxycycline (5 mg/kg, PO, q 12 h) ≥ 5 days prior to urine collection. Urine samples from 2 of these dogs were positive 113 and 120 days after initiating a 14-day course of doxycycline. Both dogs were fully vaccinated against Leptospira spp following initial diagnosis and did not have clinical signs of leptospirosis at the time of urine collection. The species-specific PCR assay was performed for all 7 non–species specific PCR assay-positive samples sent to the CDC; 1 sample (kidney) from the household cluster was positive for L kirschneri, and the other 6 samples (5 urine and 1 blood) from the main outbreak were positive for L interrogans.
Table 1—
Days postonset (DPO) of clinical signs for dogs with confirmed or probable leptospirosis (n = 52*) between January 1, 2016, and June 30, 2017, in Maricopa County, Ariz, in which blood and urine samples were collected before or after antimicrobial administration for detection of Leptospira DNA by PCR assay.
| PCR assay sample type and result | All samples (n = 105) | Samples collected prior to antimicrobial (n = 50)† | Samples collected after antimicrobial (n = 41)† | |||
|---|---|---|---|---|---|---|
| No. (%) | Median (range) DPO | No. (%) | Median (range) DPO | No. (%) | Median (range) DPO | |
| Blood | ||||||
| Positive | 15 (29) | 2.0 (0–8)‡ | 12 (46) | 2.0 (0–8)§ | 0 (0) | 0 |
| Negative | 37 (71) | 8.0 (0–165)‡ | 14 (54) | 7.0 (0–15)§ | 19 (100) | 26.5 (3–165) |
| Urine | ||||||
| Positive | 33 (62) | 6.0 (0–128) | 18 (75) | 4.0 (0–15) | 11 (50) | 21.0 (4–128) |
| Negative | 20 (38) | 7.5 (0–165) | 6 (25) | 2.0 (0–8) | 11 (50) | 27.0 (3–165) |
4 dogs had samples collected for PCR assay at > 1 time point.
Excludes samples (n = 14) collected from 7 dogs with a record of antimicrobial prescription but without a recorded date of antimicrobial initiation.
Significantly (P < 0.01) different from each other.
Significantly (P < 0.05) different from each other.
Anti-Leptospira antibodies were detected by MAT in at least 1 serum sample from 23 of 25 confirmed and probable cases (Table 2). The sera of 4 dogs in the household cluster were evaluated with the MAT at the CDC, and the highest reacting serovar was Djasiman for all 4, but 1 dog also had an equally high titer to Bratislava; among these 4 dogs, titers of ≥ 1:200 were also noted for serogroups Bratislava (n = 4), Autumnalis (4), Grippotyphosa (4), Cynopteri (3), and Pomona (3). Of the 19 dogs from the main outbreak with detectable antibodies by MAT, 3 received a Leptospira vaccine 76 to 157 days prior to sample collection; Canicola was the highest reacting serovar for 13 of the 16 remaining dogs.
Table 2—
Microscopic agglutination titers for the highest seroreactive Leptospira serovars for MAT-positive dogs with confirmed and probable leptospirosis (n = 20)* associated with 2 outbreaks between January 1, 2016, and June 30, 2017, in Maricopa County, Ariz.
| Highest titer | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Serovar | 1:100 | 1:400 | 1:800 | 1:1,600 | 1:6,400 | 1:12,800 | 1:26,500 | 1:51,200 | 1:102,400 | Total |
| Household outbreak (n = 4) | ||||||||||
| Djasiman† | 1 | 1 | 1 | 3 | ||||||
| Djasiman† and Bratislava | 1 | 1 | ||||||||
| Main outbreak (n = 16) | ||||||||||
| Canicola | 2 | 3 | 2‡ | 2 | 1 | 3 | 13 | |||
| Mankarso† | 1§ | 1 | ||||||||
| Canicola and Ballum† | 1 | 1 | ||||||||
| Autumnalis and Icterohemorrhagiae | 1 | 1 | ||||||||
Excludes 4 seropositive MAT results (range, 1:200 to 1:1,600) from 3 dogs (2 confirmed cases and 1 probable case) that were vaccinated against Leptospira spp 76 to 157 days prior to sample collection.
Serovar was only included in the MAT panel at the CDC.
One dog had acute and convalescent sera evaluated (seronegative 8 days and seropositive 40 days after the onset of clinical signs).
Dog was classified as a probable case on the basis of clinical signs and positive ELISA result.
At initial presentation, a diagnostic test for Leptospira infection was performed for 45% (32/71) of dogs classified as confirmed or probable cases; the number of dogs tested was not significantly (P = 0.48) different between dogs that initially presented with and without evidence of kidney disease.
Clinical presentation of and laboratory values for dogs with confirmed or probable leptospirosis—
Forty-one of 71 (58%) dogs were ≤ 3 years old (Table 3). Mean body weight was 25 kg (55 lb), with 55 (81%) dogs weighing ≥ 15 kg (P < 0.001). Fifty-six (79%) dogs classified as confirmed or probable cases met the clinical criteria; 8 (11%) had ≥ 1 clinical sign but did not meet the clinical criteria, and 7 (10%) that lacked clinical signs met the diagnostic and epidemiological criteria. The most common clinical signs at presentation were nonspecific and included anorexia (54/63 [86%]) and lethargy (46/63 [73%]; Supplementary Table S1, available at: avmajournals.avma.org/doi/suppl/10.2460/javma.258.6.616). One dog presented with only vomiting, and 2 dogs presented with only conjunctivitis. At presentation, the most common biochemical abnormalities were increased serum creatinine concentration (28/60 [47%]), hypokalemia (20/48 [42%]), and increased BUN concentration (25/61 [41%]). The most common hematologic abnormality was thrombocytopenia (20/54 [37%]). Eighteen of 35 (51%) dogs had a urine specific gravity ≤ 1.015 (median, 1.008; range, 1.001 to 1.015).
Table 3—
Characteristics of confirmed and probable cases of canine leptospirosis (n = 71) associated with 2 outbreaks between January 1, 2016, and June 30, 2017, in Maricopa County, Ariz.
| Characteristic | No. (%) |
|---|---|
| Age (y; n = 71) | |
| < 1 | 11 (15) |
| 1–3 | 30 (42) |
| 4–6 | 16 (23) |
| 7–9 | 10 (14) |
| ≥ 10 | 4 (6) |
| Breed (n = 71) | |
| Purebred | 45 (63) |
| Mixed breed | 26 (37) |
| Most common purebred* | |
| Siberian Husky | 7 (16)† |
| Labrador Retriever | 6 (13) |
| Weimaraner | 5 (11) |
| German Shepherd Dog | 3 (7) |
| Boxer | 2 (4) |
| Golden Retriever | 2 (4) |
| Pomeranian | 2 (4) |
| Shih Tzu | 2 (4) |
| Australian Cattle Dog | 2 (4) |
| Body weight (kg; n = 68) | |
| < 15 | 13 (19) |
| ≥ 15 | 55 (81) |
| Sex (n = 70) | |
| Male | 40 (57) |
| Female | 30 (43) |
| Neuter status (n = 66) | |
| Neutered | 54 (82) |
| Sexually intact | 12 (18) |
Purebred dogs also included 1 each of the following: Airedale Terrier, American Staffordshire Terrier, Beagle, Collie, English Cocker Spaniel, German Shorthaired Pointer, Greyhound, Irish Setter, Rhodesian Ridgeback, Rottweiler, Saint Bernard, Soft Coated Wheaten Terrier, Vizsla, and Whippet.
All were from the same household.
Treatment and disposition of confirmed and probable cases—
Medical records from 67 dogs included information on at least 1 prescribed antimicrobial; 60 (90%) dogs received doxycycline at some point during the course of their illness. Medical records from 53 dogs included details on doxycycline dosage and duration of administration. Almost all (52/53 [98%]) dogs were prescribed doxycycline for ≥ 14 days. Thirty (57%) of these dogs were prescribed doses of 5 to 9 mg/kg (4.1 mg/lb), and 6 (11%) dogs were prescribed doses ≥ 20 mg/kg (9.1 mg/lb). Other antimicrobials administered included oral formulations of amoxicillin (n = 16 [24%]), amoxicillin and clavulanic acid (10 [15%]), metronidazole (7 [10%]), enrofloxacin (3 [5%]), ampicillin (2 [3%]), and cephalexin (1 [1%]) and parenteral formulations of ampicillin and sulbactam (10 [15%]) and penicillin (1 [1%]).
Medical records from 70 dogs included information on hospitalization. Twenty-nine (41%) dogs were hospitalized overnight (n = 14 [20%]) or during hospital business hours (15 [22%]) at some point during the course of their illness. Three (4%) dogs were euthanized because of poor prognosis (2 from the household cluster and 1 from the main outbreak).
Nested case-control investigation
Characteristics of case and control dogs—
Among the 71 confirmed and probable cases, 54 were eligible for the case-control investigation, of which 44 (82%) were included. Among these 44 case dogs, 36 were confirmed cases and 8 were probable cases; all 44 case dogs were associated with the main outbreak.
Of the completed 289 unique responses to the survey for control dog owners, 281 met the inclusion criteria. No owners reported that dogs in their households had previously had leptospirosis. The majority (n = 259 [92%]) of responses were received between late July 2017 and mid-August 2017, with 219 (78%) received 1 to 3 days following survey distribution to 7 veterinary clinics and boarding facilities. Control dogs represented 16 cities throughout Maricopa County, whereas case dogs resided in 6 cities; however, the cities of Scottsdale (case dogs, n = 32 [73%]; control dogs, 195 [69%]) and Phoenix (4 [9%]; 46 [16%]) were the most common residences for both case and control dogs. Case dogs were significantly (P = 0.02) younger, heavier (body weight ≥ 15 kg; P < 0.01), more likely to be male (P = 0.02), and less likely to be neutered (P = 0.01), compared with control dogs (Table 4).
Table 4—
Characteristics of confirmed and probable cases of canine leptospirosis (case; n = 36 to 44) associated with an outbreak between October 2016 and June 2017, in Maricopa County, Ariz, and of control dogs (control; 270 to 281) that also resided in Maricopa County, Ariz, and were enrolled by means of dog owner completion of a survey from July 2017 to August 2017.
| Characteristic | Case dogs | Control dogs | OR (95% CI) |
|---|---|---|---|
| Dogs in the household | n = 36 | n = 281 | |
| Mean ± SD | 2.0 ± 1.8 | 1.7 ± 1.0 | NA |
| Age (y) | n = 44 (%) | n = 276 (%) | |
| < 1 | 5 (11) | 4 (1) | 19.1 (3.6–100.1) |
| 1–3 | 23 (52) | 75 (27) | 4.7 (1.5–14.3) |
| 4–6 | 6 (14) | 64 (23) | 1.43 (0.4–5.3) |
| 7–9 | 6 (14) | 72 (26) | 1.3 (0.3–4.7) |
| ≥ 10 | 4 (9) | 61 (22) | REF |
| Dog type* | n = 42 (%) | n = 270 (%) | |
| Mixed breed | 23 (55) | 142 (53) | REF |
| Herding | 6 (14) | 12 (4) | 3.2 (1.0–9.2) |
| Hound | 3 (7) | 6 (2) | 3.3 (0.8–13.8) |
| Nonsporting | 0 (0) | 20 (8) | 0.148 (0.0008–2.7) |
| Sporting | 7 (17) | 28 (10) | 1.6 (0.6–4.0) |
| Terrier | 1 (2) | 26 (10) | 0.3 (0.1–1.9) |
| Toy | 1 (2) | 28 (10) | 0.3 (0.1–1.8) |
| Working | 1 (2) | 8 (3) | 1.1 (0.2–7.0) |
| Weight (kg) | n = 44 (%) | n = 281 (%) | |
| < 15 | 13 (30) | 147 (52) | REF |
| ≥ 15 | 31 (70) | 134 (48) | 2.6 (1.3–5.2) |
| Sex | n = 44 (%) | n = 280 (%) | |
| Female | 16 (36) | 157 (56) | REF |
| Male | 28 (64) | 123 (44) | 2.2 (1.1—4.2) |
| Neuter status | n = 44 (%) | n = 280 (%) | |
| Sexually intact | 6 (14) | 12 (4) | REF |
| Neutered | 38 (86) | 268 (96) | 0.3 (0.1–0.8) |
| Dog role† | n = 44 (%) | n = 281(%) | |
| Pet | 43 (98) | 279 (99) | NA |
| Hunting | 1 (2) | 1 (< 1) | NA |
| Guard | 1 (2) | 8 (3) | NA |
| Service or emotional support | 1 (2) | 2 (< 1) | NA |
| Show | 0 (0) | 1 (< 1) | NA |
Purebred dogs were assigned to American Kennel Club–recognized breed groups.
Categories not mutually exclusive. NA = Not applicable. REF = Reference group for calculation of OR.
Exposure assessment—
The most common exposures for case dogs 30 days before the onset of clinical signs or, for dogs lacking clinical signs, date of diagnosis were visits to dog daycare facilities (59%), grooming facilities (30%), and dog parks (30%) and overnight stays at boarding facilities (30%; Table 5). After adjusting for age, body weight, sex, neuter status, and median household income, dogs that visited dog daycare facilities or stayed overnight at boarding facilities were 7.7 (95% CI, 3.5 to 16.7) and 2.9 (95% CI, 1.3 to 6.6) times as likely to be a case, respectively, as those that did not. Dogs with a history of travel outside their city of residence (adjusted OR, 0.19; 95% CI, 0.04 to 0.59) or hiking (adjusted OR, 0.2; 95% CI, 0.05 to 0.76) were significantly less likely to be a case than those without a history of travel or hiking. After adjustment, dogs of the high- and moderate-exposure groups for dog daycare facilities were 13.8 (95% CI, 5.2 to 36.9) and 4.6 (95% CI, 1.8 to 12.0) times as likely to be a case, respectively, compared with dogs of the low-exposure group (Table 6). Dogs that spent approximately 50% of their time outdoors were 13.2 times (adjusted OR; 95% CI, 1.6 to 636.3) as likely to be a case, compared with dogs that were always indoors, but a specific outdoor location did not significantly increase the odds of being a case in the adjusted analyses (Table 7). Dogs that had contact with rodents (adjusted OR, 7.2; 95% CI, 2.2 to 23.6) or that resided in homes in which rodents were seen (adjusted OR, 5.1; 95% CI, 1.3 to 20.7) had significantly greater odds of being a case.
Table 5—
Locations or activities that may have led to exposure to Leptospira spp within 30 days prior to the onset of clinical signs or, for dogs lacking clinical signs, 30 days prior to the date of diagnosis for the dogs of Table 5.
| Location or activity | No. of case dogs (%) | No. of control dogs* (%) | Unadjusted OR (95% CI) | Adjusted OR (95% CI) |
|---|---|---|---|---|
| Dog daycare facility | 26 (59) | 42 (15) | 8.2 (4.3–16.2) | 7.7 (3.5–16.7)†‡ |
| Dog park | 13 (30) | 42 (15) | 2.3 (1.1–4.9) | 1.5 (0.67–3.3)‡ |
| Boarding facility | 13 (30) | 39 (14) | 2.6 (1.2–5.4) | 2.9 (1.3–6.6)†‡ |
| Grooming facility | 13 (30) | 115 (41) | 0.61 (0.3–1.2) | 0.74 (0.35–1.6)‡ |
| Pet store | 9 (20) | 71 (25) | 0.76 (0.35–1.7) | 0.48 (0.21–1.1)‡ |
| Drank from standing water§ | 8 (18) | 36 (13) | 1.5 (0.65–3.5) | 1.3 (0.52–3.3)∥ |
| Travel¶ | 4 (9) | 91 (32) | 0.22 (0.05–0.62) | 0.19 (0.04–0.59)†∥ |
| Hiking | 3 (7) | 44 (16) | 0.39 (0.12–1.3) | 0.2 (0.05–0.76)†‡ |
| Swam or played in water or mud§ | 2 (5) | 26 (9) | 0.47 (0.05–2.0) | 0.18 (0.02–1.1)‡ |
| Obedience school facility | 2 (5) | 11 (4) | 1.16 (0.12–5.6) | 0.75 (0.14–4.0)† |
| Dog show | 0 (0) | 3 (1) | — | — |
Denominator varied among location or activity: dog daycare, n = 280; dog park and dog show, 278; boarding and grooming facilities, pet store, drank from standing water, travel, and hiking, 281; and swam or played in water or mud and obedience school facility, 279.
OR was significantly (P < 0.05) different from 1.0.
Adjusted for age, body weight, sex, neuter status, and median household income of the owner’s US Census Bureau tract.
Excluded swimming pool.
Adjusted for age, body weight, sex, and neuter status.
Outside of the city of residence. — = Not determined.
Table 6—
Frequency of visits to dog daycare facilities and dog parks over 30 days for the dogs of Table 4.
| Exposure | Frequency* | No. of case dogs (%) | No. of control dogs (%) | Unadjusted OR (95% CI) | Adjusted OR (95% CI)† |
|---|---|---|---|---|---|
| Dog daycare facility | High | 16 (36) | 14 (5) | 15.1 (6.4–35.8) | 13.8 (5.2–36.9) |
| Moderate | 10 (23) | 28 (10) | 4.7 (2.0–8.9) | 4.6 (1.8–12.0) | |
| Low | 18 (41) | 238 (85) | REF | REF | |
| Dog park | High | 8 (18) | 19 (7) | 3.2 (1.3–7.9) | 2.4 (0.89–6.7) |
| Moderate | 5 (11) | 23 (8) | 1.7 (0.59–4.7) | 0.9 (0.28–2.7) | |
| Low | 31 (70) | 236 (85) | REF | REF |
High = ≥ 1/wk. Moderate = ≥ 1/30 d but < 1/wk. Low = No known potential exposure.
Adjusted for age, body weight, sex, neuter status, and median household income of the owner’s US Census Bureau tract.
REF = Reference group for calculation of unadjusted and adjusted ORs.
Table 7—
Lifestyle factors for the dogs of Table 4.
| Lifestyle factor | No. of case dogs* (%) | No. of control dogs† (%) | Unadjusted OR (95% CI) | Adjusted OR‡ (95% CI) |
|---|---|---|---|---|
| Home area | ||||
| Urban or suburban | 43 (98) | 275 (98) | REF | REF |
| Rural, farm, or ranch | 1 (2) | 6 (2) | 1.1 (0.02–2.5) | 0.98 (0.02–12.2) |
| Water feature§ at home | ||||
| Yes | 9 (21) | 54 (19) | 1.1 (0.5–2.5) | 1.3 (0.54–2.9) |
| No | 33 (79) | 224 (81) | REF | REF |
| Water bowl location | ||||
| Inside home | 38 (88) | 251 (89) | REF | REF |
| Inside and outside home | 2 (5) | 22 (8) | 0.60 (0.07–2.6) | 0.45 (0.05–2.1) |
| Outside home | 3 (7) | 8 (3) | 2.5 (0.4–10.9) | 1.8 (0.28–8.2) |
| Food bowl location | ||||
| Inside home | 41 (95) | 272 (97) | REF | REF |
| Inside and outside home | 0 (0) | 2 (1) | — | — |
| Outside home | 2 (5) | 7 (2) | 1.9 (0.19–10.4) | 1.2 (0.1–7.8) |
| Indoor-outdoor time | ||||
| Always outdoors | 0 (0) | 1 (< 1) | — | — |
| 50% indoors-50% outdoors | 9 (21) | 27 (10) | 17.5 (2.2–803.1) | 13.2 (1.6–636.3)∥ |
| Mostly indoors | 33 (77) | 199 (71) | 8.9 (1.4–370.9) | 7.3 (1.1–315.5)∥ |
| Always indoors | 1 (2) | 54 (19) | REF | REF |
| Location when outdoors¶ | ||||
| Fenced yard | 31 (70) | 222 (79) | 0.63 (0.31–1.3) | 0.66 (0.30–1.4) |
| Leashed walk | 29 (66) | 183 (65) | 1.0 (0.53–2.0) | 0.97 (0.47–2.0) |
| Dog park | 12 (27) | 36 (13) | 2.6 (1.2–5.4) | 1.8 (0.80–4.1) |
| Hiking trail | 1 (2) | 33 (12) | 0.18 (0.004–1.1) | 0.08 (0.002–0.55)∥ |
| Roams outdoors | 2 (5) | 9 (3) | 1.4 (0.15–7.3) | 0.94 (0.09–5.5) |
| Park (other) | 1 (2) | 12 (4) | 0.5 (0.01–3.7) | 0.49 (0.01–3.8) |
| Contact with rodents | 8 (18) | 8 (3) | 7.5 (2.3–24.5) | 7.2 (2.2–23.6) ∥ |
| Contact with livestock | 2 (5) | 4 (1) | 3.3 (0.29–23.6) | 2.5 (0.22–28.5) |
| Contact with wildlife | 4 (9) | 7 (3) | 2.0 (1.04–3.75) | 1.9 (0.94–3.92) |
| Rodents seen inside home | 4 (9) | 8 (3) | 3.5 (1.0–12.0) | 5.1 (1.3–20.7)∥ |
| Rodents seen on property | 19 (44) | 81 (30) | 1.9 (1.9–3.6) | 2.0 (0.96–4.1) |
Denominator varied among lifestyle factors for case dogs: water bowl location, food bowl location (inside or outside) home, indoor-outdoor time, contact with wildlife, rodents seen inside the home, and rodents seen on the property, n = 43; and food bowl location (inside and outside house), 42.
Denominator varied among lifestyle factors for control dogs: a water feature at home and rodents seen inside home, n = 278; contact with livestock, 280; contact with wildlife, 277; and rodents seen on property, 271.
Adjusted for age, body weight, sex, and neuter status.
Included swimming pool.
OR was significantly (P < 0.05) different from 1.0.
Responses were recorded as yes or no; dog owners could select > 1 location.
REF = Reference group for calculation of unadjusted and adjusted ORs.
— = Not determined.
Leptospirosis vaccination—
No case dogs had documented vaccination (2-dose series) against Leptospira spp prior to the onset of clinical signs. Three case dogs that were confirmed by PCR assay received their first dose of Leptospira vaccine in response to a boarding facility cluster (part of the main outbreak) 3, 13, or 15 days prior to the onset of clinical signs. At the time of survey completion, 93 of 281 (33%) owners of control dogs reported that their dogs had received a Leptospira vaccine at some point. A sensitivity analysis that included 136 dogs whose owners responded that their dogs had never received a Leptospira vaccine revealed that calculated point estimates moved away from the null hypothesis (that dogs that had more regular and frequent contact with other dogs or were frequently in areas where dogs congregated were not at greater odds for infection) and therefore did not affect the outcome of the main analysis. Among owners whose dogs were not vaccinated, the most commonly cited reasons were the owner “did not know there was a vaccine” (25%), the “veterinarian did not recommend the vaccine” (20%), the dog was “not considered at risk for leptospirosis by the veterinarian” (19%), and owner fear of an adverse reaction (10%).
Discussion
To the authors’ knowledge, this investigation was the first to characterize the clinical, diagnostic, and epidemiological features of and risk factors for a community-wide, urban-suburban outbreak of canine leptospirosis in a low-prevalence area. Epidemiological and diagnostic laboratory data indicated 2 outbreaks: a cluster of 8 dogs in 1 household in early 2016 in which L kirschneri was identified with a species-specific PCR assay and in which Djasiman was the most frequent highest reacting serovar with MAT, and a community-wide outbreak affecting 63 dogs from the fall of 2016 to the spring of 2017 in which L interrogans was identified and Canicola was the most frequent highest reacting serovar. Although the source of each outbreak was not confirmed, contact between unvaccinated dogs and Leptospira-infected dogs or exposure to contaminated environments where dogs congregated likely allowed for propagation of the outbreak.
Knowledge of an infecting Leptospira serovar can help to identify an animal reservoir, a source of infection, and the risk factors for future infection. However, identification of the infecting serovar requires isolation of the Leptospira spp via culture, which is notoriously challenging because Leptospira spp are slow-growing, fastidious bacteria; unsurprisingly, bacteria were not isolated from dogs in the present investigation. Although MAT results are reported as antibody titers to serovars representing serogroups, antibodies to specific serovars frequently cross-react with other serovars. Studies23–27 in various species and geographic locations reveal that the highest reacting MAT serovar does not reliably predict the infecting serovar, with reported sensitivities of 33% to 96% on the basis of various titer cutoffs. Noteworthy, however, is that MAT titers for infected dogs from the main outbreak in the present investigation were highly consistent, with 13 of 16 dogs never previously vaccinated having had the highest titer to Canicola. Even allowing for the limitations in the sensitivity of MAT, this population-level pattern suggests it is likely that a serovar from the Canicola serogroup infected some dogs in the main outbreak. Across the United States, however, the prevalence of serovar Canicola is thought to be decreasing, presumably because of effective vaccines that include Canicola antigen.15,28,29 Low preoutbreak vaccination rates in Maricopa County may have been a reason for infections with serogroup Canicola.
The consistency in MAT results among 4 dogs from the household cluster and their shared environment suggested that all 4 dogs were infected with the same serovar of Leptospira. Serogroup Djasiman is not a commonly reported cause of canine leptospirosis, possibly in part because it is not commonly included on MAT panels offered by veterinary diagnostic laboratories in the United States. However, cross-reactions are common, and even an MAT panel with a limited number of serovars could still detect cross-reactive antibodies to an infecting serovar not included with a particular laboratory’s panel. Because of the potential for cross-reactions,26,30 we cannot discern whether a serovar from the serogroup Djasiman or from one of the other serogroups to which dogs had antibodies, including those more commonly included on MAT panels (eg, Bratislava, Autumnalis, Grippotyphosa, and Pomona), was the infecting serovar. A sample from 1 dog in the household cluster was confirmed with L kirschneri; this species includes 1 serovar from the Djasiman serogroup31 as well as other serovars to which these dogs reacted, including Grippotyphosa, a serovar commonly reported to cause infections of dogs.
Diagnosis of leptospirosis by PCR assay is impacted by the timing of sample collection for testing and antimicrobial administration. The time during which leptospires are detected in the blood is short, often < 1 week, and although shedding of leptospires in the urine can persist for weeks to months, shedding occurs intermittently31; therefore, a negative PCR assay result should never be considered as sufficient evidence to rule out infection. Leptospira DNA was most commonly detected in urine samples, likely a reflection of the relatively short duration of leptospiremia, compared with leptospiruria. Additionally, the majority of dogs were not tested for Leptospira infection at initial presentation, thereby increasing the interval between the onset of clinical signs and sample collection. Whole blood samples positive for Leptospira DNA were collected significantly earlier after the onset of clinical signs (median, 2 days) versus negative samples (median, 8 days). Antimicrobial administration prior to blood and urine sample collection can result in a negative PCR assay result and therefore a missed diagnosis.1,20 In the present investigation, blood samples were more sensitive to prior antimicrobial administration than urine samples, with Leptospira DNA detected in 11 of 22 (50%) urine samples. Despite these findings, veterinarians should strive to collect both blood and urine samples prior to initiating antimicrobial administration to improve the likelihood of obtaining a positive PCR assay result for infected dogs.1,20
The length of time during which an infected dog’s urine can contain viable leptospires after antimicrobial treatment has important implications for the management of infected dogs, because infected dogs and their urine can contaminate the environment and can infect people and other dogs. Resources1,2,18 commonly suggest that urine shedding of leptospires ceases after the first few days of antimicrobial treatment; however, this precept likely originated from rodent models32 and early experimental canine models33 performed before PCR assays were available. Finding Leptospira DNA in 11 dogs’ urine samples that were collected between 4 and 128 days after initiating antimicrobial administration makes this precept questionable. Without growth of Leptospira spp via culture, however, discerning whether the detected DNA represents viable leptospires is impossible; yet a positive PCR assay result indicated that these dogs had the potential to still have been shedding viable leptospires. For 2 of these 11 dogs, the recommended 14-day regimen of doxycycline failed to eliminate leptospiruria, with Leptospira DNA detected in their urine samples collected > 3 months later and with resolution of clinical signs for 1 dog (the other dog did not have clinical signs at the time of diagnosis). Given the expected short-term natural immunity following infection34 and the fact that both dogs were fully vaccinated against Leptospira spp after diagnosis, reinfection was unlikely. More likely, these dogs were persistently shedding serogroup Canicola leptospires, for which dogs are the reservoir hosts. One case series34 and 1 case report35 also indicate persistent leptospiruria despite antimicrobial treatment. These collective findings highlight the need to research the duration of urine shedding of leptospires of different species and serovars after antimicrobial treatment so that appropriate infection control can be recommended.1
Most dogs did not have clinical signs or had only mild nonspecific signs (eg, lethargy, anorexia, vomiting, or diarrhea) of leptospirosis. Heightened awareness of the main outbreak and therefore increased testing of dogs with known exposure to a dog with confirmed leptospirosis, or of dogs that had been kenneled at a boarding facility, might have resulted in a detection bias in which dogs with mild, nonspecific clinical signs or subclinical infections that might have otherwise gone undiagnosed were included in the present investigation. This inclusion may have then resulted in a higher survival rate (68/71 [96%]) than commonly reported.1 Only 30% (19/63) of dogs from the main outbreak initially presented with fever, highlighting that fever is not necessary for considering leptospirosis as a differential diagnosis.2 Seven dogs did not have clinical signs of leptospirosis, and 3 dogs presented with only conjunctivitis, which may not usually prompt testing for leptospirosis. Although the infecting serovar might have influenced the severity of the observed clinical signs, the frequency of mild, nonspecific signs in this outbreak supported the conclusion that canine leptospirosis is likely under-recognized and underdiagnosed. Although cases of canine leptospirosis have been rarely reported in Arizona, a 2015 survey36 of 298 Arizona veterinarians revealed that during 2011 to 2015, 34 cases of leptospirosis in any species had been diagnosed by respondents, suggesting that dogs were diagnosed but diagnoses were not reported to the state veterinarian.
The finding that dogs that visited dog daycare facilities or were kenneled overnight at dog boarding facilities had greater odds of being a case (with greater odds for high-exposure group dogs vs moderate-exposure group dogs, when compared with low-exposure group dogs) was consistent with the reports of case clusters associated with dog daycare and boarding facilities during the fall of 2016 and winter of 2017. However, detection bias was possible if ill dogs that had been kenneled at dog boarding facilities were more likely to be seen by a veterinarian and subsequently tested for Leptospira infection. Dog parks have the potential for both wildlife access and the persistence of Leptospira spp in the environment. Although nearly one-third of case dogs had visited a dog park 30 days before the onset of clinical signs, dog park visitation did not significantly increase the odds of being a case in the adjusted model.
Travel to Florida and Southern California was reported for 1 dog from the household cluster of show dogs in the 30 days prior to the onset of clinical signs. However, for the main outbreak, travel in the prior 30 days was significantly less commonly reported by owners of case dogs than by those of control dogs; this finding could be because dogs that traveled with their owners were removed from the outbreak location and therefore unlikely to be kenneled at boarding facilities or taken to area dog daycare facilities. Similarly, hiking was found to be protective in the adjusted model, although only 16% (44/281) of owners of control dogs reported hiking with their dogs. Yet hiking could be an alternative to dog daycare and dog parks as a means to meet a dog’s exercise needs and might reduce exposure to areas where dogs congregate. Additionally, although the location of hiking was not recorded, hiking in Maricopa County is likely to be in dry, desert environments where water is less likely to pool than in landscaped or irrigated yards. However, these observed associations may have been influenced by recall bias. Because of delays in obtaining contact information for owners of case dogs, interviews were conducted weeks to months after diagnosis, and owners were asked to recall exposures in the 30 days prior to diagnosis, whereas owners of control dogs were asked about the 30 days prior to survey completion. Therefore, activities that may have occurred sporadically, such as hiking or drinking from standing water, might have been underestimated for case dogs.
Historically, contact with wildlife, livestock, and freshwater sources, rather than dog-to-dog transmission, has been considered one of the most important risk factors for the development of leptospirosis.1 Although the odds of being a case were significantly greater for dogs that frequently visited dog daycare or boarding facilities, we do not know whether direct dog-to-dog transmission had occurred or naïve dogs had come in contact with Leptospira-contaminated environments at these facilities. Regardless of the manner of transmission, frequency of contact with other dogs and exposure to environments where dogs congregate are not commonly examined as risk factors for leptospirosis. Instead, to the authors’ knowledge, published risk-based algorithms for whether dogs should be vaccinated against Leptospira spp focus on geography and the dog’s likelihood of contact with animals other than dogs or an environmental source of leptospires.37 On the basis of the geographic area and possible risk factors identified in the present investigation, expansion of the criteria for recommending Leptospira vaccination should be considered.
Because of differences in the age, body weight, and sex of case and control dogs, these factors were controlled for as potential confounders in the multivariable models. Purebred dogs were overrepresented in the case series, compared with mixed-breed dogs, but this finding was likely influenced by the inclusion of 7 infected Siberian Huskies of the household cluster. In the case-control investigation, no breed was significantly more likely to be a case, compared with mixed-breed dogs. Although some studies reveal that dogs of a certain sex (male),38–41 type (herding,39,42 hound,39,42 mixed-breed,38 and working dogs38,39,43), or age (< 1 year,38 4 to 6.9 and 7 to 9.9 years,39 5 to 10 and > 10 years,42 and 4 to 6.9 years44) are at increased risk for developing leptospirosis, findings are not consistent. Furthermore, other studies do not reveal any age,40 breed,40,44 or sex predilection.43 Likely, associations between these characteristics and the risk of developing leptospirosis vary with the sources of infection; possibly, age, breed, or body weight impacts the chance a dog will be exposed to a leptospire-contaminated environment. For example, owners of young or large-breed dogs may be more likely to have them at dog daycare facilities so that they can expend energy, thus impacting the effect of age or body weight on outbreaks associated with dog daycare facilities. Ultimately, a dog of any age, breed, or sex can be at risk for developing leptospirosis when exposed to an infected animal or contaminated environment.
Despite the increased odds of being a case for a dog that visited a dog daycare facility or was kenneled overnight for boarding, animals other than dogs may have played a role in the initiation or propagation of the outbreak. Dogs had greater odds of being a case when they spent time outdoors (vs always indoors), regardless of location. Although dogs that had contact with rodents were 7 times as likely to be a case as those that did not, contact was reported by only 18% (8/44) of owners of case dogs. Urban coyotes and foxes that could maintain serovar Canicola thrive in Arizona, and anti-Leptospira antibodies have been detected in coyotes,45,46 rodents,47 and collared peccaries.48 Therefore, potential wildlife Leptospira reservoirs exist in Arizona. Convenience sampling and testing of culled wildlife could be used to better establish whether a local wildlife reservoir specifically exists in Maricopa County, and results could be used to better understand the prevalence of leptospirosis there.
The present investigation was limited by reliance on passive surveillance and reporting; however, communications from personnel at affected facilities and various county and state agencies to dog owners, veterinarians, and personnel at dog daycare and boarding facilities about leptospirosis likely resulted in diagnostic testing and reporting that might not have occurred otherwise. Yet given the potential for nonspecific clinical signs, additional cases associated with the outbreak were likely not reported, and if characteristics for reported cases substantially differed from those for unreported cases, results may not have been representative of the outbreak. Additionally, information contained in the medical records was not standardized among veterinary clinics and therefore was limited to that routinely recorded by clinic personnel and resulted in missing data for some dogs.
Additional limitations were associated with the timing and recruitment of control dogs. Recruitment of control dogs did not occur at the time of the outbreak, such that the evaluated 30-day period was that prior to the completion of the survey for control dogs (July 2017 to August 2017) versus prior to the onset of clinical signs for case dogs (October 2016 to June 2017). To mitigate this limitation, we also assessed the control dogs’ lifestyle for regularly occurring exposures over the previous 6 months. Although recruitment for control dogs relied on owner self-selection, the similar distribution of case and control dogs by city suggested that control dogs were recruited largely through clinics that had reported outbreak cases. Also, because clinical signs of leptospirosis can be mild and nonspecific and Leptospira infection was not ruled out for control dogs, some control dogs may have had a subclinical infection and then may have been subsequently misclassified; however, we would have expected this misclassification to bias calculated point estimates toward the null hypothesis, such that they would have been underestimated and weak associations for some lifestyle factors might not have been identified.
We chose not to include Leptospira vaccination as a potential protective factor against the development of leptospirosis in the case-control analysis because vaccination occurred concurrent to the outbreak, with some dog boarding facilities having begun to require vaccination following a recommendation by the state veterinarian and distribution of educational materials to veterinary and animal care communities. Although owners of control dogs were asked whether their dog had ever been vaccinated against Leptospira spp, information to determine whether the initial 2-dose series and booster doses of vaccine had been administered prior to or during the outbreak was not available. If a large proportion of control dogs had been adequately vaccinated against Leptospira spp in the 6 months before inclusion in the present investigation, vaccination could have acted as a confounder, such that the magnitude of calculated ORs for various lifestyle factors and activities might have been less or significant ORs might have been masked. This limitation was addressed through a sensitivity analysis in which case dogs were compared with a subset of control dogs that had never been vaccinated; the sensitivity analysis supported the findings of the main analysis.
For the present investigation, we described a multiagency response to a community-wide outbreak of leptospirosis in dogs. The detection of and response to infectious disease outbreaks among companion animals is limited by an imperfect system in which jurisdictional gaps between agricultural, veterinary health, and public health agencies can result in no single agency with a clear mandate to investigate or respond to these outbreaks. For zoonotic companion animal diseases, veterinary reports can alert public health authorities to existing or possible human cases. Additionally, local, state, and federal public health personnel have expertise in disease outbreak investigation and response and may be a resource to those investigating outbreaks of companion animal zoonotic diseases. Collaboration between animal and human health agencies is essential to address the threat of emerging and reemerging zoonotic diseases.
The present investigation revealed that exposure to areas where dogs congregate, such as dog daycare and boarding facilities, may put dogs at greater odds of developing Leptospira infection in addition to commonly established risk factors. In this outbreak, low preoutbreak rates of Leptospira vaccination likely permitted propagation of infection in these areas. These findings indicated that dogs can be at risk for developing Leptospira infection, even in geographic regions where disease prevalence is thought to be low. In addition to exposure to rodents, wildlife, livestock, and natural freshwater sources, veterinarians should consider dog-contact lifestyle factors, such as at dog daycare and boarding facilities, when recommending vaccination against Leptospira spp. Veterinarians and dog owners should also consider that infected dogs may not immediately cease urinary shedding of viable leptospires after antimicrobial treatment and therefore may continue to pose a risk for the spread of infection to people and other animals.
Supplementary Material
Acknowledgments
No external funding was used in this investigation. The authors declare that there were no conflicts of interest.
The findings and conclusions in this article are those of the authors and do not necessarily represent the official position of the CDC.
The authors thank the following for their contributions to this investigation: Kristine Bisgard, Ken Komatsu, Kevin Speigel, Viacony Herzi, Elizabeth Dooley, Byron Robinson, Dana Haberling, and Marissa Person.
ABBREVIATIONS
- MAT
Microscopic agglutination test
Appendix
Clinical, diagnostic, and epidemiological criteria used to classify cases of dogs with illness suspected to be caused by Leptospira spp between January 1, 2016, and June 30, 2017, whose owners’ primary residence was in Maricopa County, Ariz, and reported by veterinarians, owners of dog daycare or boarding facilities, and dog owners to the state veterinarian or state or local public health departments.*
| Clinical criteria 1,18–20 | ||
| ≥ 2 from the following list: | or | ≥ 1 from the following list: |
|
|
| Diagnostic criteria 13–17 | Confirmatory—positive PCR assay result from any clinical specimen or a confirmatory MAT titer or a 4-fold rise in MAT titer in an unvaccinated dog or a dog vaccinated ≥ 2 months prior to sample collection. |
| Supportive—supportive MAT titer or positive IgM-IgG point-of-care test or ELISA result. |
| Vaccination status relative to diagnostic sample collection† | Confirmatory criteria MAT titer | Supportive criteria MAT titer | Detection of combined IgM-IgG anti-Leptospira antibodies via point-of-care test or ELISA |
|---|---|---|---|
| Not vaccinated | ≥ 1:400 | ≥ 1:200 but < 1:400 | Yes (meets criteria) |
| Vaccinated ≥ 6 months; or unknown vaccination status; or vaccinated but date of vaccination unknown | ≥ 1:800 | ≥ 1:400 but < 1:800 | Yes (meets criteria) |
| Vaccinated 3–6 months | ≥ 1:6,400 | ≥ 1:1,600 but < 1:6,400 | No (does not meet criteria) |
| Vaccinated 0–3 months | ≥ 1:12,800 | ≥ 1:6,400 but < 1:12,800 | No (does not meet criteria) |
| Epidemiological criteria | Dog that was exposed to another dog in the household with confirmed leptospirosis, was kenneled or attended daycare at a facility where a dog with confirmed leptospirosis had also been, or had direct contact with a dog with confirmed leptospirosis, as reported by the dog’s owner |
Confirmed case = Met confirmatory diagnostic criteria. Probable case = Met ≥ 2 of the 3 criteria: clinical, supportive diagnostic, or epidemiological. Dogs that did not meet the clinical criteria were classified as probable cases only if they had never been vaccinated against Leptospira spp. Suspect case = Met clinical criteria alone or supportive diagnostic criteria alone but did not meet any other case definition. Not a case = No anti-Leptospira antibodies detected by means of MAT or IgM-IgG point-of-care test or ELISA in serum collected ≥ 10 days after illness onset.
Because serologic tests for Leptospira spp cannot differentiate between naturally occurring and vaccine-induced antibodies, diagnostic criteria for quantitative and qualitative serologic tests varied on the basis of time since vaccination and expected persistence of IgM and IgG antibodies.
Footnotes
Excel, Microsoft Corp, Redmond, Wash.
SNAP Lepto Test, Idexx, Westbrook, Me.
Leptospira spp antibody by ELISA—canine, Idexx, Westbrook, Me.
Qualtrics, Provo, Utah.
SAS, version 9.4, SAS Institute Inc, Cary, NC.
References
- 1.Sykes JE, Hartmann K, Lunn KF, et al. 2010 ACVIM small animal consensus statement on leptospirosis: diagnosis, epidemiology, treatment, and prevention. J Vet Intern Med 2011;25:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Goldstein RE. Canine leptospirosis. Vet Clin North Am Small Anim Pract 2010;40:1091–1101. [DOI] [PubMed] [Google Scholar]
- 3.Broughton ES, Scarnell J. Prevention of renal carriage of leptospirosis in dogs by vaccination. Vet Rec 1985;117:307–311. [DOI] [PubMed] [Google Scholar]
- 4.Schreiber P, Martin V, Najbar W, et al. Prevention of renal infection and urinary shedding in dogs by a Leptospira vaccination. Vet Microbiol 2005;108:113–118. [DOI] [PubMed] [Google Scholar]
- 5.Barragan V, Olivas S, Keim P, et al. Critical knowledge gaps in our understanding of environmental cycling and transmission of Leptospira spp. Appl Environ Microbiol 2017;83:e01190–e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.White AM, Zambrana-Torrelio C, Allen T, et al. Hotspots of canine leptospirosis in the United States of America. Vet J 2017;222:29–35. [DOI] [PubMed] [Google Scholar]
- 7.Smith AM, Arruda AG, Evason MD, et al. A cross-sectional study of environmental, dog, and human-related risk factors for positive canine leptospirosis PCR test results in the United States, 2009 to 2016. BMC Vet Res 2019;15:412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ward MP. Seasonality of canine leptospirosis in the United States and Canada and its association with rainfall. Prev Vet Med 2002;56:203–213. [DOI] [PubMed] [Google Scholar]
- 9.Guagliardo SAJ, Iverson SA, Reynolds L, et al. Despite high-risk exposures, no evidence of zoonotic transmission during a canine outbreak of leptospirosis. Zoonoses Public Health 2019;66:223–231. [DOI] [PubMed] [Google Scholar]
- 10.Dikken H, Kmety E. Serological typing methods of leptospires. In: Bergan T, Norris JR, eds. Methods in microbiology. London: Academic Press, 1978;259–307. [Google Scholar]
- 11.Galloway RL, Hoffmaster AR. Optimization of LipL32 PCR assay for increased sensitivity in diagnosing leptospirosis. Diagn Microbiol Infect Dis 2015;82:199–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ferreira AS, Costa P, Rocha T, et al. Direct detection and differentiation of pathogenic Leptospira species using a multi-gene targeted real time PCR approach. PLoS One 2014;9:e112312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Martin LER, Wiggans KT, Wennogle SA, et al. Vaccine-associated Leptospira antibodies in client-owned dogs. J Vet Intern Med 2014;28:789–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Idexx Reference Laboratories. Diagnosing and managing canine leptospirosis. Available at: www.idexx.com/files/canine-leptospirosis-test-dx-update.pdf. Accessed Feb 9, 2018.
- 15.Stokes JE, Kaneene JB, Schall WD, et al. Prevalence of serum antibodies against six Leptospira serovars in healthy dogs. J Am Vet Med Assoc 2007;230:1657–1664. [DOI] [PubMed] [Google Scholar]
- 16.Miller MD, Annis KM, Lappin MR, et al. Variability in results of the microscopic agglutination test in dogs with clinical leptospirosis and dogs vaccinated against leptospirosis. J Vet Intern Med 2011;25:426–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.CDC National Notifiable Diseases Surveillance System. Leptospirosis (Leptospira interrogans) 2013 case definition. Available at: wwwn.cdc.gov/nndss/conditions/leptospirosis/case-definition/2013/. Accessed Feb 14, 2017.
- 18.Greene CE, Sykes JE, Brown CA, et al. Leptospirosis. In: Greene CE, ed. Infectious diseases of the dog and cat. 3rd ed. St Louis: Elsevier, 2006;402–417. [Google Scholar]
- 19.Adin CA, Cowgill LD. Treatment and outcome of dogs with leptospirosis: 36 cases (1990–1998). J Am Vet Med Assoc 2000;216:371–375. [DOI] [PubMed] [Google Scholar]
- 20.Schuller S, Francey T, Hartmann K, et al. European consensus statement on leptospirosis in dogs and cats. J Small Anim Pract 2015;56:159–179. [DOI] [PubMed] [Google Scholar]
- 21.Haake DA, Levett PN. Leptospirosis in humans. Curr Top Microbiol Immunol 2015;387:65–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.US Census Bureau. American community survey. Available at: www.factfinder.census.gov. Accessed Oct 3, 2017.
- 23.Smythe LD, Wuthiekanun V, Chierakul W, et al. The microscopic agglutination test (MAT) is an unreliable predictor of infecting Leptospira serovar in Thailand. Am J Trop Med Hyg 2009;81:695–697. [DOI] [PubMed] [Google Scholar]
- 24.Harkin KR, Hays MP. Variable-number tandem-repeat analysis of leptospiral DNA isolated from canine urine samples molecularly confirmed to contain pathogenic leptospires. J Am Vet Med Assoc 2016;249:399–405. [DOI] [PubMed] [Google Scholar]
- 25.Blanco RM, dos Santos LF, Galloway RL, et al. Is the microagglutination test (MAT) good for predicting the infecting serogroup for leptospirosis in Brazil? Comp Immunol Microbiol Infect Dis 2016;44:34–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Levett PN. Usefulness of serologic analysis as a predictor of the infecting serovar in patients with severe leptospirosis. Clin Infect Dis 2003;36:447–452. [DOI] [PubMed] [Google Scholar]
- 27.Lizer J, Velineni S, Weber A, et al. Evaluation of 3 serological tests for early detection of Leptospira-specific antibodies in experimentally infected dogs. J Vet Intern Med 2018;32:201–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gautam R, Wu CC, Guptill LF, et al. Detection of antibodies against Leptospira serovars via microscopic agglutination tests in dogs in the United States, 2000–2007. J Am Vet Med Assoc 2010;237:293–298. [DOI] [PubMed] [Google Scholar]
- 29.Goldstein RE, Lin RC, Langston CE, et al. Influence of infecting serogroup on clinical features of leptospirosis in dogs. J Vet Intern Med 2006;20:489–494. [DOI] [PubMed] [Google Scholar]
- 30.Chirathaworn C, Inwattana R, Poovorawan Y, et al. Interpretation of microscopic agglutination test for leptospirosis diagnosis and seroprevalence. Asian Pac J Trop Biomed 2014;4(suppl 1):S162–S164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Levett PN. Leptospirosis. Clin Microbiol Rev 2001;14:296–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Truccolo J, Charavay F, Merien F, et al. Quantitative PCR assay to evaluate ampicillin, ofloxacin, and doxycycline for treatment of experimental leptospirosis. Antimicrob Agents Chemother 2002;46:848–853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yoder HW, Bergman EN, Gleiser CA. Experimental canine leptospirosis IV: evaluation of selected antibiotics in the therapy of acute experimental Leptospira icterohemorrhagiae infections in immature dogs. J Infect Dis 1957;100:257–267. [DOI] [PubMed] [Google Scholar]
- 34.Mauro T, Harkin K. Persistent leptospiruria in five dogs despite antimicrobial treatment (2000–2017). J Am Anim Hosp Assoc 2019;55:42–47. [DOI] [PubMed] [Google Scholar]
- 35.Prescott J. Urinary shedding of spirochaetes in a dog with acute leptospirosis despite treatment. Vet Rec 2011;169:187–188. [DOI] [PubMed] [Google Scholar]
- 36.Venkat H, Yaglom HD, Adams L. Knowledge, attitudes, and practices relevant to zoonotic disease reporting and infection prevention practices among veterinarians — Arizona, 2015. Prev Vet Med 2019;169:104711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.American Animal Hospital Association. Lifestyle-based vaccine calculator. Available at: www.aaha.org/guidelines/canine_vaccination_guidelines/vaccine_calculator.aspx. Accessed May 15, 2018.
- 38.Azócar-Aedo L, Monti G. Meta-analyses of factors associated with leptospirosis in domestic dogs. Zoonoses Public Health 2016;63:328–336. [DOI] [PubMed] [Google Scholar]
- 39.Ward MP, Glickman LT, Guptill LF. Prevalence of and risk factors for leptospirosis among dogs in the United States and Canada: 677 cases (1970–1998). J Am Vet Med Assoc 2002;220:53–58. [DOI] [PubMed] [Google Scholar]
- 40.Rentko VT, Clark N, Ross LA, et al. Canine leptospirosis — a retrospective study of 17 cases. J Vet Intern Med 1992;6:235–244. [DOI] [PubMed] [Google Scholar]
- 41.Ward MP, Guptill LF, Wu CC. Evaluation of environmental risk factors for leptospirosis in dogs: 36 cases (1997–2002). J Am Vet Med Assoc 2004;225:72–77. [DOI] [PubMed] [Google Scholar]
- 42.Hennebelle JH, Sykes JE, Foley J. Risk factors associated with leptospirosis in dogs from northern California: 2001–2010. Vector Borne Zoonotic Dis 2014;14:733–739. [DOI] [PubMed] [Google Scholar]
- 43.Delaude A, Rodriguez-Campos S, Dreyfus A, et al. Canine leptospirosis in Switzerland—a prospective cross-sectional study examining seroprevalence, risk factors and urinary shedding of pathogenic leptospires. Prev Vet Med 2017;141:48–60. [DOI] [PubMed] [Google Scholar]
- 44.Ward MP, Guptill LF, Prahl A, et al. Serovar-specific prevalence and risk factors for leptospirosis among dogs: 90 cases (1997–2002). J Am Vet Med Assoc 2004;224:1958–1963. [DOI] [PubMed] [Google Scholar]
- 45.Drewek J, Noon TH, Trautman RJ, et al. Serologic evidence of leptospirosis in a southern Arizona coyote population. J Wildl Dis 1981;17:33–37. [DOI] [PubMed] [Google Scholar]
- 46.Grinder M, Krausman PR. Morbidity-mortality factors and survival of an urban coyote population in Arizona. J Wildl Dis 2001;37:312–317. [DOI] [PubMed] [Google Scholar]
- 47.Songer JG, Chilelli CJ, Reed RE, et al. Leptospirosis in rodents from an arid environment. Am J Vet Res 1983;44:1973–1976. [PubMed] [Google Scholar]
- 48.Corn JL, Lee RM, Erickson GA, et al. Serologic survey for evidence of exposure to vesicular stomatitis virus, pseudorabies virus, brucellosis and leptospirosis in collared peccaries from Arizona. J Wildl Dis 1987;23:551–557. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


