Figure 1.
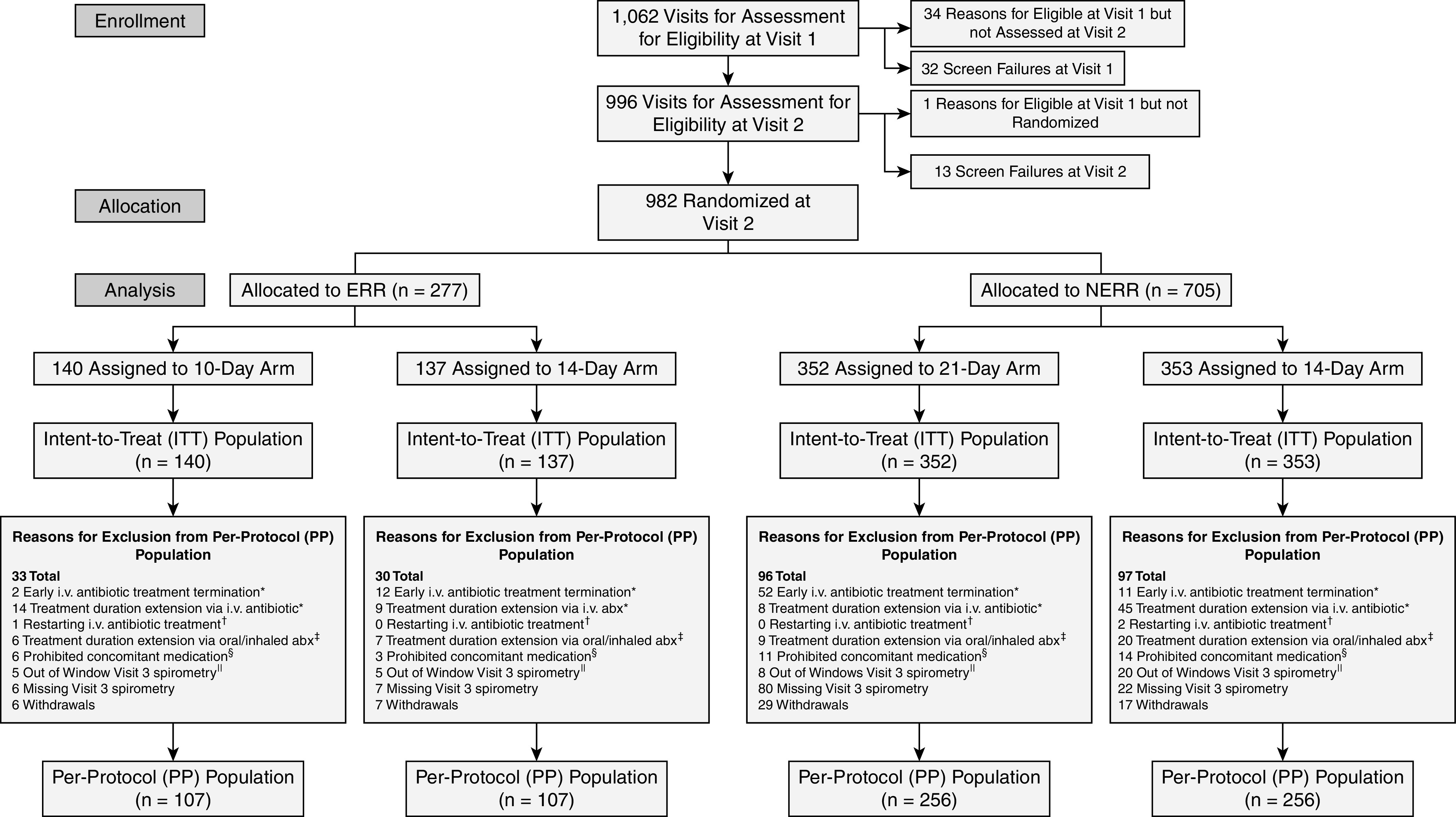
Consolidated Standards of Reporting Trials (CONSORT) diagram. Note: A participant can be excluded from the PP population for more than one reason. Exclusions due to protocol violations: *i.v. antibiotic treatment ending outside the allowed window for a deviation from assigned treatment duration: ± 1 day for 10- and 14-day assignments, ± 3 days for 21-day assignments; †restarting i.v. antibiotic treatment 1 or 2 days after the actual completion of i.v treatment; ‡any acute oral or inhaled antibiotic started before the scheduled completion of i.v treatment and continued after the scheduled completion of i.v. treatment; §any acute oral or inhaled antibiotic started after the scheduled completion of the i.v. treatment and before Visit 3, except continuation of ongoing chronic treatment of initiation of new chronic antibiotic therapy; and ∥spirometry measures done 4 or more days outside of the allowed window for Visit 3 (± 2 days). abx = antibiotics; ERR = early robust responder; NERR = non–early robust responder.
