Figure 2.
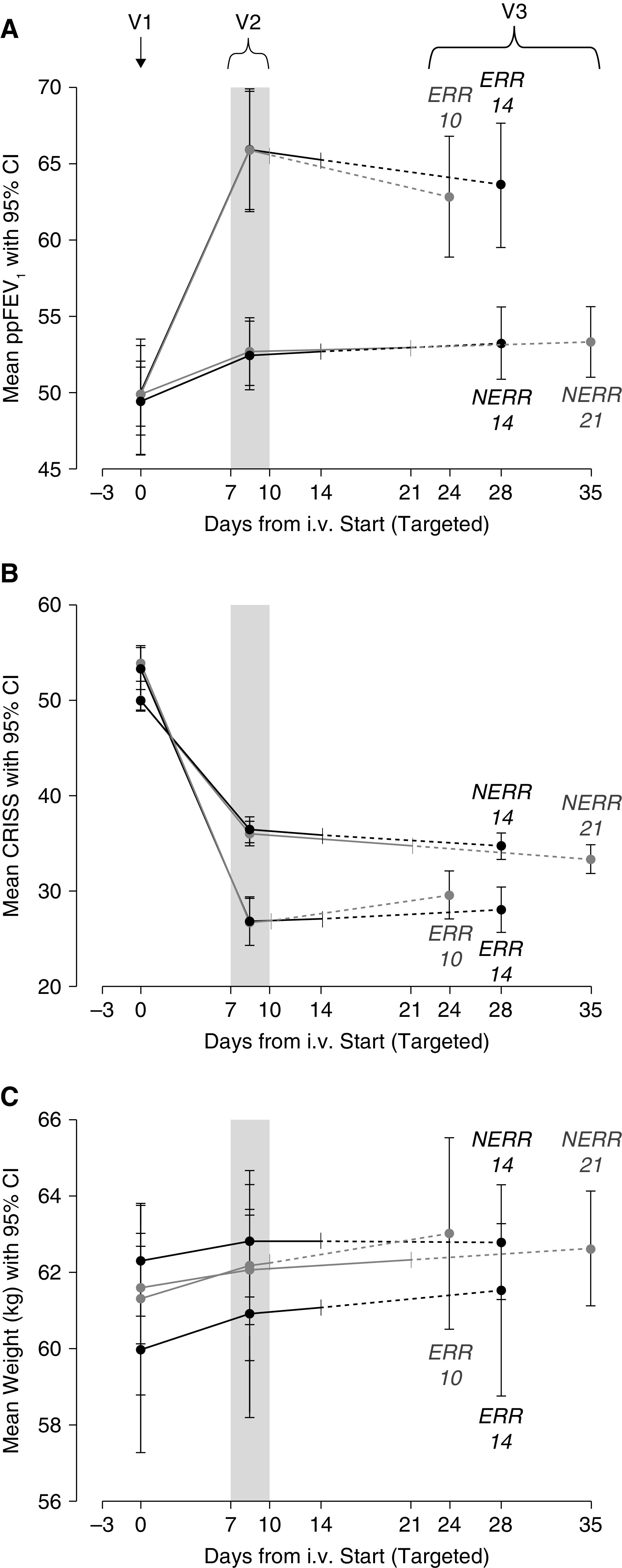
Outcomes by visit, allocation, and antimicrobial treatment duration: (A) Primary outcome ppFEV1. (B) Chronic respiratory infection symptom score (CRISS). (C) Weight. V1 = visit 1 at start of intravenous antimicrobials; V2 = visit 2 when randomization occurs; and V3 = visit 3 was targeted for 14 days after scheduled end of intravenous antimicrobial treatment. Table E1 in the online supplement provides realized timing of each visit by allocation and duration assignment. CI = confidence interval; ERR = early robust responder; NERR = non–early robust responder; ppFEV1 = percent of predicted FEV1.
