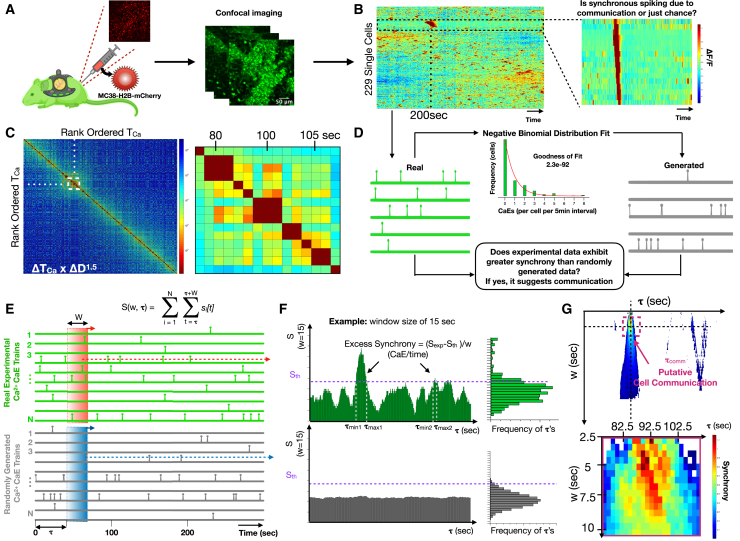
Figure 3.
Csf1r-GCaMP5 reporter cell communication in vivo revealed by intravital imaging through a dorsal window chamber
(A) Cartoon illustration of the intravital imaging of a calcium reporter mouse with a dorsal window chamber and orthotopically injected MC38-H2B-mCherry tumor cells.
(B) Heatmap of single-cell fluorescence dynamics hierarchically clustered with single cells (y axis) versus time (x axis) where color represents the normalized change in fluorescence ΔF/F. Inset shows a cluster of cells with temporally localized calcium elevations.
(C) Cross-correlation heatmap of rank-ordered calcium elevations for all cells. Color represents the product of calcium elevation time difference and Euclidean spatial distance ΔTOCaE × ΔD1.5. Inset shows the region of high cross-correlation indicating calcium elevations that are highly localized in time and space.
(D) Strategy for constructing generated (simulated) cells sampled from the same negative binomial distribution of calcium elevation trains as the “real” experimental calcium elevation trains. Comparisons allow estimation of whether synchrony occurs by chance or exhibits excess synchrony, beyond chance, which we interpret as putative cell communication.
(E) Method for quantifying normalized number of calcium elevations (S/w), also known as synchrony, for real and generated single-cell calcium elevation trains, where S is a function of temporal window size, w, and window initiation time, τ.
(F) Method for defining the timing of excess synchrony of real cell populations compared with the corresponding generated populations.
(G) Heatmap of excess synchrony (ΔS/w) as a function of temporal window size, w, and window initiation time, τ. Inset shows timing of high excess synchrony and putative cell communication.