Figure 6. mRAPD3 competes with RVFV glycoprotein Gn for binding to Lrp1 and inhibits RVFV infection.
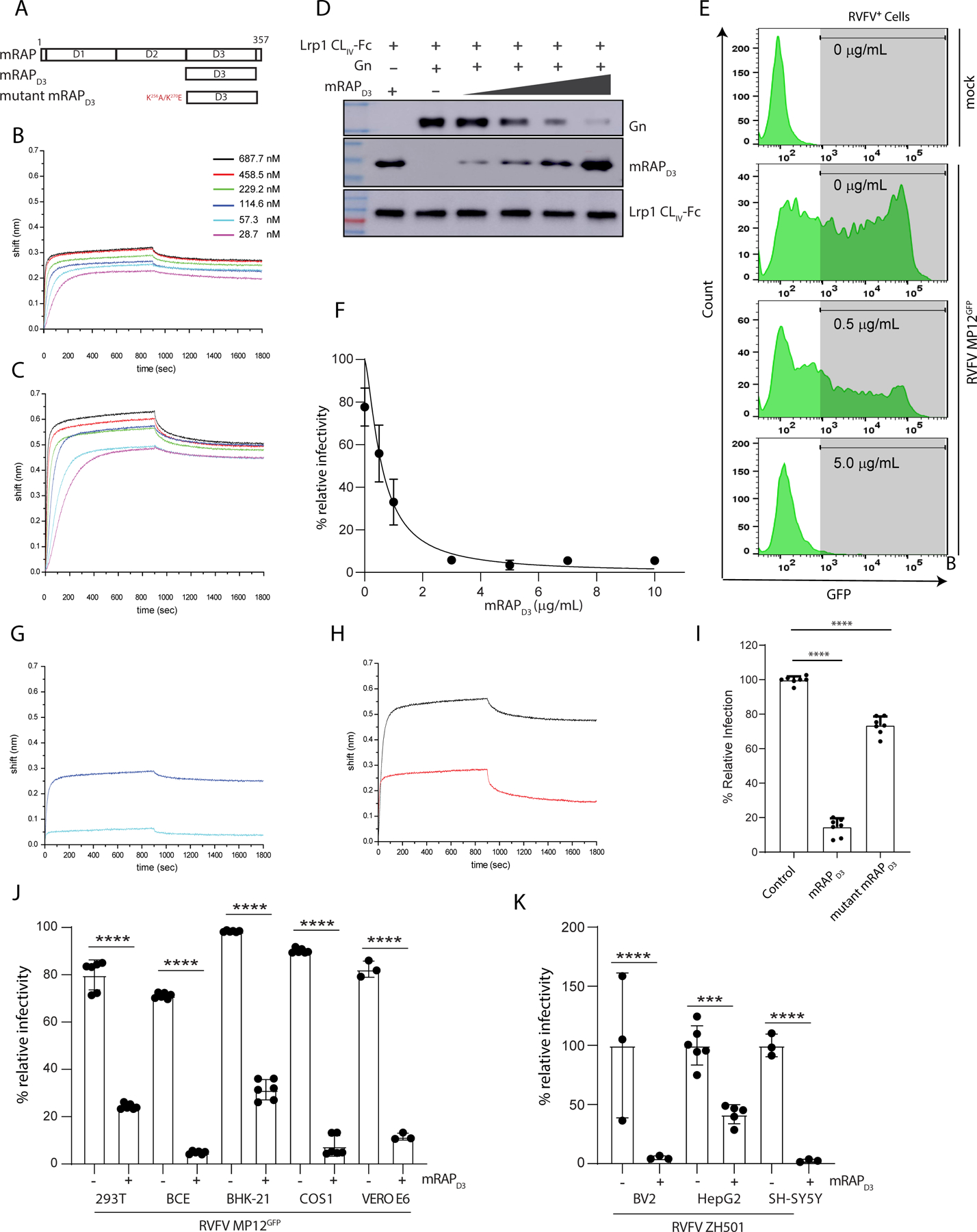
A. Domain organization of mouse RAP (mRAP) protein. BLI sensograms of mRAPD3 binding to immobilized B. LRP1 CLII immobilized C. LRP1 CLIV. D. mRAPD3 competition assay to assess relative binding of Gn to LRP1 CLIV in the presence of 1, 3, 6, or 10 μg/mL concentrations of mRAPD3. E. Flow cytometry data for BV2 cells infected with RVFV MP12GFP in the presence of increasing concentrations of mRAPD3. F. Analysis of relative infectivity as a function of mRAPD3 concentration. EC50 is 0.59 ± 0.2 μg/ml. BLI sensograms showing the binding of mRAPD3 and mutant mRAPD3 with G. LRP1 CLII and H. LRP1 CLIV. I. RVFV MP12GFP infection of BV2 cells in presence of mRAPD3 and mutant mRAPD3. J. Cell lines from different species were infected with RVFV-MP12GFP at an MOI 1 in the absence (−) or presence (+) of 5 μg/mL of mRAPD3 (10x EC50). Infection was assessed 15 hpi by flow cytometry. K. Mouse (BV2) and human (HepG2 and SH-SY5Y) cell lines were infected with RVFV ZH501 at an MOI 1 in the absence (−) or presence (+) of mRAPD3. Infection was assessed at 18 hpi by RT-qPCR on cell supernatants and intracellular flow cytometry for viral Gn protein.
