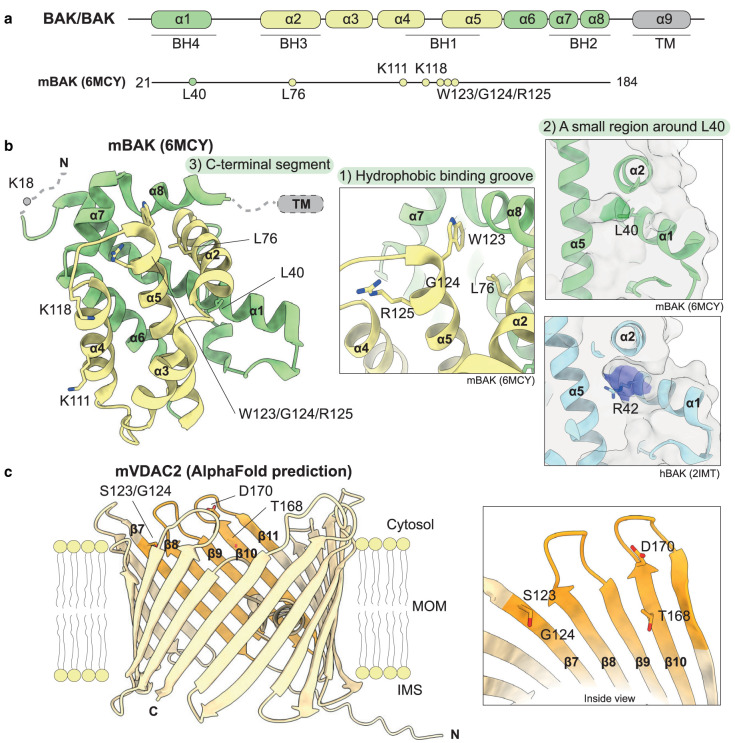
Figure 2. Potential interface on BAK and VDAC2 involved in VDAC2–BAK interaction.
(a) Diagram of pro-apoptotic effector proteins BAX and BAK with proposed VDAC2 interacting residues on mouse BAK (mBAK). (b) The crystal structure of mBAK (PDB: 6MCY) [61] is shown in cartoon representation with α-helices 2 to 5 forming the hydrophobic surface groove shown in yellow; residues implicated in the VDAC2–BAK interaction are shown as sticks. The proposed interacting residues on BAK can be classified into three hot spots: (1) the BAK canonical hydrophobic binding grove formed by α-helices 2–5; (2) a small region behind the canonical binding groove centred around L40 in mBAK (green) which is equivalent to R42 in human BAK (hBAK, cyan, PDB: 2IMT) [9]; (3) the BAK TM domain (not resolved in this structure) and the C-terminus of α8. (c) AlphaFold2 predicted model of mVDAC2 (UniProt: Q60930) is shown in yellow cartoon with β7–10 highlighted in orange [62,63]. The region on VDAC2 involved in VDAC2–BAK interaction has been mapped to its β7–10 region, where T168 and D170 are considered crucial while S123 and G124 are also relevant. They all locate to the cytosol-orientated region of VDAC2, facing towards the inside of the pore.