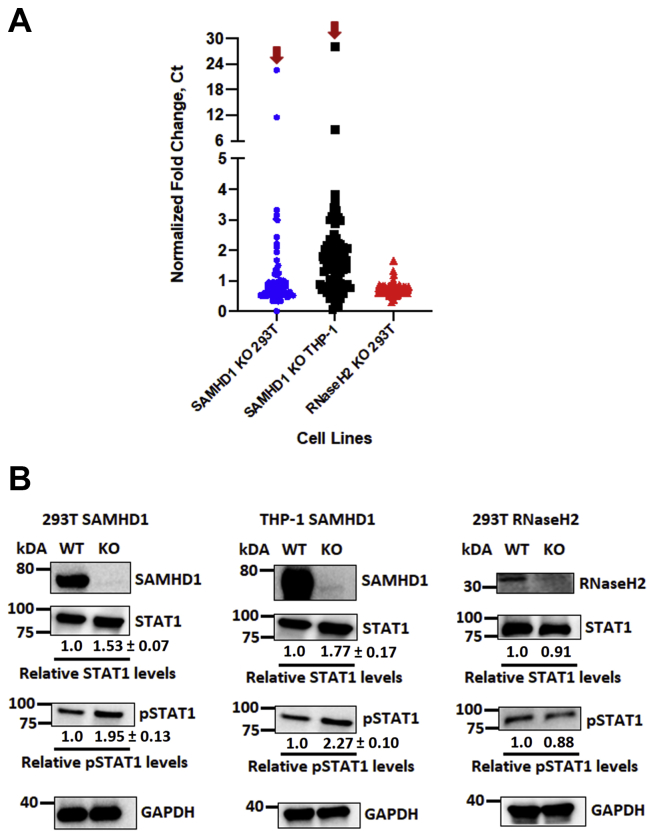
Figure 4.
Human interferon pathways genes and proteins are upregulated in the absence of SAMHD1. Total intracellular RNA isolated from SAMHD1 WT and KO 293T and differentiated THP-1 macrophages as well as RNaseH2 WT and KO 293T cells was used for random cDNA fragment syntheses. The resulting cDNA samples were utilized to evaluate human interferon gene expression using the TaqMan Array Human Interferon Pathway, Fast 96-well (Thermo Fisher Scientific). A, the qPCR Ct values of each gene in SAMHD1 KO and RNaseH2 KO cells were compared and normalized with the WT cells and presented as normalized fold change computed using the Livak method (47). Four housekeeping genes embedded in the array were used for the signal normalization. Red arrows indicate STAT1 mRNA fold change in SAMHD1 KO 293T and differentiated THP-1 cells. Normalized fold changes of specific mRNAs of each cell line are presented in Figs. S3–S5. B, STAT1 and pSTAT1 protein expressions in each cell line, in the presence or the absence of SAMHD1 or RNaseH2 expression, were evaluated via Western blot using antihuman STAT1 and pSTAT1 antibodies. GAPDH was used as a loading control. The relative STAT1 and pSTAT1 protein levels were normalized with GAPDH, and the ratios between respective WT and KO cells were calculated. Relative changes in STAT1 and pSTAT1 protein expression in SAMHD1 KO cells were presented as means of triplicates ± standard deviations from the means. SAMHD1 Western blot data of 293T and differentiated THP-1 cells were from the same blots presented in Figures 1A and 2A. cDNA, complementary DNA; pSTAT1, phosphorylated form of STAT1; qPCR, quantitative PCR; SAMHD1, sterile alpha motif and histidine–aspartate domain–containing protein 1; STAT1, signal transducer and activator of transcription 1.