Abstract
Background:
A known relationship exists between oxidative stress and preterm birth (PTB). However, few studies have measured oxidative stress prospectively in early or midpregnancy, and no studies have used electron paramagnetic resonance (EPR) spectroscopy prospectively to predict PTB.
Objective:
The purpose of this study was to identify predictive relationships between antioxidants and reactive oxygen species (ROS), specifically, superoxide (), peroxynitrite (OONO−), and hydroxyl radical (●OH), using EPR spectroscopy, measured between 12 and 20 weeks of gestation and compare with the incidence of PTB.
Methods:
Blood was obtained from pregnant women (n = 140) recruited from a tertiary perinatal center. Whole blood was analyzed directly for ROS, , OONO−, and ●OH using EPR spectroscopy. Red blood cell lysate was used to measure antioxidants. PTB was defined as parturition at <37 weeks of gestation.
Results:
No differences were found between ROS, , OONO−, or ●OH with the incidence of PTB. Catalase activity, glutathione, and reduced/oxidized glutathione ratio were significantly lower with PTB. Logistic regression suggests decreased catalase activity in pregnant women is associated with increased odds of delivering prematurely.
Discussion:
We prospectively compared antioxidants and specific ROS using EPR spectroscopy in pregnant women between 12 and 20 weeks of gestation with the incidence of PTB. Results are minimal but do suggest that antioxidants—specifically decreased catalase activity—in early pregnancy may be associated with PTB; however, these findings should be cautiously interpreted and may not have clinical significance.
Keywords: catalase, preterm birth, superoxide
The economic burden of preterm birth (PTB) resulting from acute and chronic perinatal complications has prioritized PTB as a public health problem. Although this biopsychosocial phenomenon has been extensively studied, the pathophysiology of PTB remains unclear. Experts speculate the role of oxidative stress in the etiology of PTB. Oxidative stress is often defined as an imbalance between reactive oxygen species (ROS) and antioxidants resulting in conditions such as apoptosis from lipid peroxidation, protein alterations, irreversible cellular damage, and DNA oxidation (Burton & Jauniaux, 2011; Schieber & Chandel, 2014). However, oxidative stress also involves a disruption of redox-dependent signaling that contributes to the pathogenesis of disease (Jones, 2008). One of the most prominent endogenous ROS is superoxide (), a one-electron reduction of molecular oxygen generated from leakage of electrons along the mitochondria electron transport chain and from enzymes that deliberately transfer an electron from an electron donor (i.e., nicotinamide adenine dinucleotide phosphate or NADPH) to oxygen (Burton & Jauniaux, 2011). Antioxidants help achieve redox homeostasis by directly scavenging ROS and/or repairing damage induced by ROS. Common endogenous antioxidants include cellular enzymes (e.g., superoxide dismutase [SOD], catalase [CAT]) and small molecule thiols (e.g., glutathione).
Excessive ROS may be damaging, but an unstable and dysregulated redox environment is also believed to disrupt cell signaling and control pathways causing further physiological dysregulation (Jones, 2008). For example, hydrogen peroxide (H2O2) is an ROS that, in excess, can be damaging when chemically reacting with metal ions. CAT is one of the catalytic enzymes that breaks down H2O2 to maintain redox homeostasis. In a homeostatic redox environment, H2O2 is also important for signaling and activation of specific biological responses, such as cellular migration, differentiation, proliferation, and apoptosis (Veal, Day, & Morgan, 2007). Disruption of signal transduction is believed to interfere with these biological processes causing further dysregulation and damage to cellular processes associated with pathophysiological conditions.
Previous perinatal researchers have operationally defined oxidative stress by measuring antioxidant levels in various specimen sources (e.g., vaginal wash fluid, cord blood, and maternal blood) and/or quantifying damage caused from oxidative stress as discussed in a recent integrative review (Moore, Ahmad, & Zimmerman, 2018). Defining oxidative stress by the presence of a biomarker (e.g., isoprostane, hydroxydeoxyguanasine, total oxidant status) can be limited as the biomarker may or may not be causal to the pathophysiological condition, thus inhibiting the interpretation and consequently the development of therapeutic interventions (Frijhoff et al., 2015). Synthesized results of these measures are inconclusive for PTB because of inconsistent findings and variation in measurements and specimen sources (Moore et al., 2018). Notably, the gold standard for measuring specific ROS in biological samples is electron paramagnetic resonance (EPR) spectroscopy (Hawkins & Davies, 2014), which provides more specific data and quantification of ROS associated with a certain phenotype—in this case, PTB. Moreover, the existing data on associations between oxidative stress and PTB have been primarily retrospectively measured during labor, in cord blood at birth, or in the neonate (Moore et al., 2018).
Few studies have measured oxidative stress (i.e., levels of ROS and antioxidants) prospectively in early or midpregnancy, and no studies have used EPR spectroscopy prospectively to predict PTB (Moore et al., 2018; Polettini et al., 2017; Wei, Fraser, & Luo, 2010). The theoretical framework for this study is derived from McEwen’s allostatic load (McEwen, 1998), suggesting that physiological dysregulation in pregnant women increases the risk of PTB. The purpose of this secondary aim was to identify predictive relationships between oxidative stress measured between 12 and 20 weeks of gestation with PTB. The primary objectives were to identify differences and predictive relationships between specific ROS and antioxidants measured in pregnant women between 12 and 20 weeks of gestation with the incidence of term birth (TB) versus PTB.
METHODS
Human subjects’ approval was received from the institutional review board of the primary author’s university. A prospective, longitudinal design was used; a convenience sample of 140 pregnant women from a Midwest tertiary perinatal center were enrolled. Inclusion criteria included <20 weeks of gestation and the intention to deliver at the onsite labor and delivery unit. Exclusion criteria was known congenital anomalies of the fetus at time of consent. Pregnant women between 12 and 20 weeks of gestation were consecutively recruited from a maternal-fetal medicine clinic (n = 70) and a general obstetrics clinic (n = 70) within the same facility. After participants gave informed, written consent, blood samples were obtained.
Measurements
Maternal blood was collected between 12 and 20 weeks of gestation. ROS were measured in whole blood using EPR spectroscopy, as previously described (Ahmad, Temme, Abdalla, & Zimmerman, 2016; Moore et al., 2019). Briefly, within 30 minutes of blood collection, a superoxide-sensitive EPR spin probe, 1-hydroxy-3-methoxycarbonyl-2,2,5,5-tetramethylpyrrolidine (CMH), was added to whole blood in separate wells of a 24-well culture plate. Samples were incubated with CMH for 30 minutes at 37°C, frozen in liquid nitrogen, and placed into a liquid nitrogen-filled finger dewar, which was then inserted into a Bruker eScan EPR spectrometer (Bruker BioSpin GmbH, Rheinstetten/Karlsruhe, Germany). Superoxide-mediated oxidation of the CMH results in the formation of a stable nitroxide radical (CM•) that can be detected by EPR spectroscopy. The amplitude of the EPR spectrum that is generated by the stable nitroxide is directly proportional to the concentration of the free radical with which it reacted (Dikalov, Griendling, & Harrison, 2007). To determine the specific levels of ROS in the collected blood samples, one well of the 24-well plate included CMH alone (ROS), one well included CMH + SOD protein to primarily identify the presence of superoxide (), one well included CMH + uric acid to primarily identify the presence of peroxynitrite (OONO−), and one well included CMH + dimethylthiourea (DMTU) to primarily identify the presence of hydroxyl radical (●OH). To interpret the EPR results for the wells with added scavengers, a decrease in the EPR spectrum amplitude in samples containing SOD, uric acid, or DMTU, as illustrated in Figure 1, strongly indicates , OONO−, or ●OH (respectively) was present in the sample. The amplitude of the spectrum is expressed as EPR arbitrary units.
FIGURE 1.
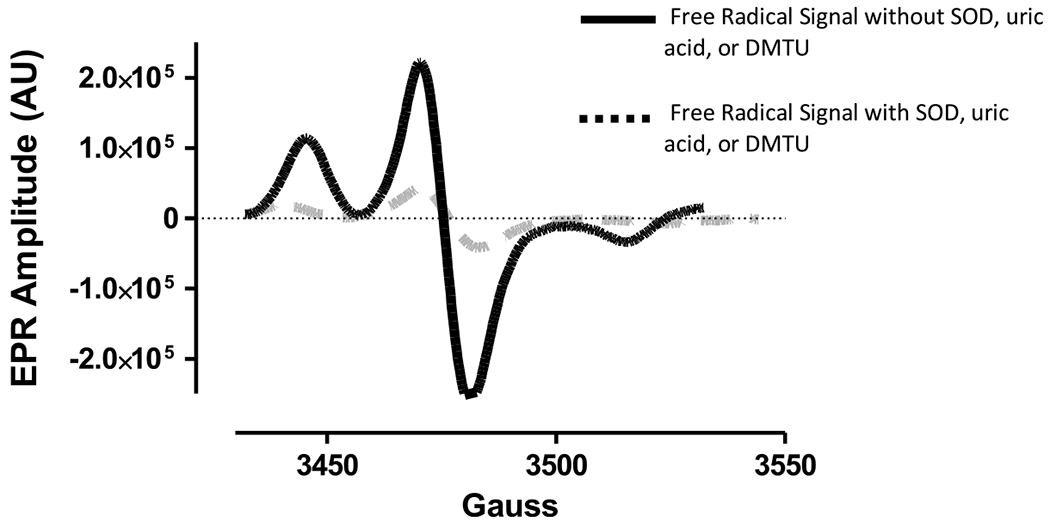
Electron paramagnetic resonance signal with/without reactive oxygen species scavengers.
The remaining blood was centrifuged to separate plasma from cellular components, divided into aliquots, frozen, and stored in a −80°C freezer for batch processing. The red blood cell pellet was lysed and used to measure CAT activity using the OxiSelect Catalase Activity Assay Kit (Cell Biolabs, Inc., San Diego, CA), SOD activity using SOD Assay Kit-WST (DOJINDO, Inc., Rockville, MD), and reduced and oxidized glutathione (GSH and GSSG, respectively) using the GSSG/GSH Quantification kit (DOJINDO, Inc., Rockville, MD). All assays were performed using the manufacturer’s instructions.
Statistical Analyses
Medians and interquartile ranges were calculated separately for women with TB versus PTB. Differences between TB and PTB were studied using Mann-Whitney U tests because the data were not normally distributed. A Bonferroni correction was used to account for multiple testing; thus, statistical significance was identified as a p < .005. A multivariable logistic regression model predicting TB/PTB was completed using the log of , OONO−, ●OH, CAT, SOD, and GSH/GSSG; GSH, GSSG, and CMH were not included due to the multicollinearity with GSH/GSSG and the other ROS scavengers (SOD, uric acid, or DMTU), respectively. The data were log-transformed to account for skewed data in the regression model. SPSS v25 (SPSS Inc., Chicago, IL) was used for analyses.
RESULTS
Pregnant women (N = 140) were consecutively recruited from a Midwest tertiary perinatal center between August 2014 and October 2016. Most of the participants were White (67%) and non-Hispanic (88%) and had private insurance (53%). The mean maternal age was 28.8 years (range: 18–42 years). Twenty-eight of the pregnant women had some type of diabetes mellitus: Type I (n = 5), Type II (n = 6), and gestational diabetes (n = 17). Seventeen of the pregnant women were diagnosed with preeclampsia. Sixty-four of the women had scheduled inductions, and the majority were vaginal deliveries (n = 93). Of these demographic variables, diabetes mellitus status and preeclampsia were significantly associated with PTB (p = .013 and p ≤ .001, respectively). Table 1 shows differences in maternal oxidative stress measurements between 12 and 20 weeks of gestation for the women with an outcome of TB (n = 106) and PTB (n = 24). Missing data were due to miscarriages, withdrawals, transfer of care to another birth center, and limited sample volumes. As noted in Table 1, no significant differences in the EPR amplitude from blood samples containing ROS, , ONOO−, and ●OH in TB compared to PTB were found. CAT activity (p ≤ .001), GSH (p ≤ .001), and GSH/GSSG (p = .004) were lower in PTB compared to TB.
TABLE 1.
Differences Between Maternal Parameters of Oxidative Stress With Term Birth or Preterm Birth Outcomes
| Term |
Preterm |
|||||
|---|---|---|---|---|---|---|
| n b | Median (P25, P75) | n b | Median (P25, P75) | p a | ||
| A.U. | ROS (CMH only) | 98 | 8.3 × 105 (5.8 × 105, 1.0 × 106) | 17 | 9.4 × 105 (5.8 × 105, 1.1 × 106) | .696 |
| CMH + SOD | 95 | 8.0 × 105 (6.1 × 105, 1.1 × 106) | 17 | 7.9 × 105 (5.5 × 105, 1.0 × 106) | .375 | |
| CMH + uric acid | 95 | 7.8 × 105 (6.4 × 105, 1.1 × 106) | 17 | 8.1 × 105 (5.9 × 105, 1.2 × 106) | .764 | |
| CMH + DMTU | 93 | 7.7 × 105 (5.6 × 105, 1.2 × 106) | 17 | 7.5 × 105 (5.0 × 105, 9.0 × 105) | .472 | |
| U/ml | CAT | 100 | 5.1 × 104 (2.0 × 104, 8.2 × 104) | 19 | 1.6 × 104 (1.4 × 104, 2.9 × 104) | <.001 |
| SOD | 99 | 8.6 × 104 (5.2 × 104, 1.5 × 105) | 19 | 1.7 × 104 (2.9 × 104, 1.1 × 105) | .025 | |
| nmol/ml | GSH | 98 | 1.7 × 103 (1.4 × 103, 2.1 × 103) | 19 | 1.2 × 103 (1.0 × 103, 1.5 × 103) | <.001 |
| GSSG | 98 | 34.4 (23.5, 47.4) | 19 | 41.4 (31.9, 63.9) | .340 | |
| GSH/GSSG | 98 | 52.0 (35.9, 80.3) | 19 | 23.7 (17.0, 41.0) | .004 | |
Note. A.U. = arbitrary units; ROS = reactive oxygen species; CMH = 1-hydroxy-3-methoxycarbonyl-2,2,5,5-tetramethylpyrrolidine; SOD = superoxide dismutase; DMTU = dimethylthiourea; CAT = catalase; GSH = reduced glutathione; GSSG = oxidized glutathione.
Mann–Whitney U with Bonferroni correction (p < .005).
Varied from limited or missing specimens.
Table 2 describes the adjusted odds ratios for the biomarker variables in the logistic regression model predicting PTB. Although trends were noted, only CAT was statistically significant in the model. Specifically, after adjusting for the other biomarkers in the model, a one-unit decrease in the log of CAT activity measured at 12–20 weeks of gestation in pregnant women is associated with an increased adjusted odds (AOR = .271, 95% CIs [.104, .703], p = .007) of delivering prematurely. Because diabetes status and preeclampsia are confounding variables in PTB, a separate regression model predicting PTB that included diabetes status, preeclampsia, and the log of CAT was analyzed; CAT maintained significance (AOR = .353, 95% CIs [.159, .783], p = .01).
TABLE 2.
Logistic Regression Model of Oxidative Stress Variables Predicting Preterm Birth
| Adjusted odds ratio | 95% CI | Significance | |
|---|---|---|---|
| Log CMH + SOD | 0.481 | 0.058, 3.960 | .496 |
| Log CMH + uric acid | 3.656 | 0.562, 23.802 | .175 |
| Log CMH + DMTU | 0.456 | 0.128, 1.620 | .225 |
| Log CAT | 0.271 | 0.104, 0.703 | .007 |
| Log SOD | 0.480 | 0.211, 1.089 | .079 |
| Log GSH/GSSG | 0.504 | 0.244, 1.041 | .064 |
Note. CMH = 1-hydroxy-3-methoxycarbonyl-2,2,5,5-tetramethylpyrrolidine; SOD = superoxide dismutase; DMTU = dimethylthiourea; CAT = catalase; GSH = reduced glutathione; GSSg = oxidized glutathione.
DISCUSSION
We measured whole-blood levels of ROS, , ONOO−, ●OH, circulating cellular enzymatic activity of specific endogenous antioxidants (i.e., CAT and SOD), and small molecule antioxidants (i.e., GSH and GSSG) from pregnant women between 12 and 20 weeks of gestation. No significant differences between ROS, , ONOO−, or ●OH amplitudes measured using EPR spectroscopy were found between TB and PTB. Levels of antioxidants differed for women who had TB compared to women with PTB. Specifically, levels of CAT activity, GSH, and GSH/GSSG were lower in PTB in the univariate analysis. After adjusting for multiple variables in a logistic regression, only CAT activity was significantly associated with a small change in the odds of PTB.
We did not find previous literature on studies that have used EPR spectroscopy prospectively to measure ROS, , ONOO−, or ●OH in early pregnancy and compare with the incidence of PTB. Previous research is conflicting with CAT activity and PTB. For example, differences in CAT activity measured in maternal blood at birth and cord blood were not identified in previous studies (Abiaka & Machado, 2012; Lázár, Orvos, Szőllősi, & Varga, 2015; Soydinç et al., 2012, 2013). Our results do support a study authored by Soydinc et al. (2013) in which decreased CAT activity in vaginal wash fluid was associated with PTB.
Considering CAT directly scavenges H2O2, one possible interpretation of our data is that PTB may involve excessive levels of H2O2. Although H2O2 is important in redox regulation and signaling, an abundance in the redox environment disrupts cell signaling and control contributing to further pathophysiological damage from an unstable redox environment (Sies, 2017). Unfortunately, we did not measure H2O2 levels in our samples as the CMH spin probe used in our study reacts very poorly with H2O2. Nevertheless, our EPR results in the regression model do suggest higher levels of ●OH (CMH + DMTU), which may be a result of elevated H2O2 reacting with iron (i.e., Fenton chemistry). That said, these interpretations must be considered highly speculative and cautious conclusions that require additional studies to be confirmed.
Another potential pathophysiological mechanism based on our data would be the redox potential of GSH/GSSG. In our univariate statistics, the GSH and GSH/GSSG levels were significantly lower in PTB. Under normal conditions, the reduced form of glutathione, or GSH, is more prevalent than the oxidized form, GSSG. Lower GSH and GSH/GSSG levels in our study suggests the redox environment in pregnant women who deliver preterm was more oxidative, interpreted as higher levels of oxidative stress. However, this relationship only trended toward significance when included in the logistic regression. These results do warrant further consideration because of the important role of the glutathione system in the redox potential and cell signaling and control (Jones, 2002).
Limitations
Our study has several limitations. First, we did not separately analyze data based on medical risk categories or include any other demographic factors in the regression analysis. We acknowledge that differences in oxidative stress may be affected by medical risk status, age, and other factors. Second, the a priori power analysis used for this study was based on a different primary aim (Moore et al., 2019) and, thus, may not have sufficient power to detect significance for this secondary aim. Finally, our antioxidant enzyme measurement was specific to activity and did not measure actual enzyme protein levels or other enzymes specific to the glutathione system (i.e., glutathione reductase, glutathione peroxidase), which should be considered for future studies.
CONCLUSION
In summary, we prospectively compared antioxidants and ROS, , ONOO−, and ●OH, using EPR spectroscopy, inpregnant women between 12 and 20 weeks of gestation with the incidence of PTB. Results are minimal but do suggest decreased antioxidants in early pregnancy may be associated with PTB; however, these findings should be cautiously interpreted and may not have clinical significance. Future research should examine the activity of the entire glutathione system, as well as directly measure H2O2 and hydroperoxides to identify if the dysregulation includes adequate protein, inactivity, excessive ROS, and/or insufficient antioxidant levels.
Acknowledgments
Research reported in this publication was supported by the National Institute of Nursing at the National Institutes of Health under Award Number K01NR014474 and a University of Nebraska Medical Center’s Edna Ittner Pediatric Support grant. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
This study received institutional review board approval from the University of Nebraska Medical Center: IRB 794-15-EP and 154-14-EP.
The authors wish to thank the staff and patients at Nebraska Medicine and Olson Center, Jocelyn Jones, BS, Colton T. Roessner, BS, and Ellen Steffensmeier, RN, DNP.
Footnotes
The authors report no conflict of interest.
Contributor Information
Tiffany A. Moore, College of Nursing, University of Nebraska Medical Center, Omaha.
Kaeli Samson, Department of Biostatistics, College of Public Health, University of Nebraska Medical Center, Omaha.
Iman M. Ahmad, Department of Medical Imaging and Therapeutic Sciences, College of Allied Health Professions, University of Nebraska Medical Center, Omaha.
Adam J. Case, Department of Cellular and Integrative Physiology, College of Medicine, University of Nebraska Medical Center, Omaha.
Matthew C. Zimmerman, Department of Cellular and Integrative Physiology, College of Medicine, University of Nebraska Medical Center, Omaha.
REFERENCES
- Abiaka C, & Machado L (2012). Nitric oxide and antioxidant enzymes in venous and cord blood of late preterm and term omani mothers. Sultan Qaboos University Medical Journal, 12, 300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmad IM, Temme JB, Abdalla MY, & Zimmerman MC (2016). Redox status in workers occupationally exposed to long term low levels of ionizing radiation: A pilot study. Redox Report, 21, 139–145. doi: 10.1080/13510002.2015.1101891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton GJ, &Jauniaux E (2011). Oxidative stress. Best Practice & Research. Clinical Obstetrics & Gynaecology, 25, 287–299. doi: 10.1016/j.bpobgyn.2010.10.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dikalov S, Griendling KK, & Harrison DG (2007). Measurement of reactive oxygen species in cardiovascular studies. Hypertension, 49, 717–727. doi: 10.1161/01.HYP.0000258594.87211.6b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frijhoff J, Winyard PG, Zarkovic N, Davies SS, Stocker R, Cheng D, … Ghezzi P (2015). Clinical relevance of biomarkers of oxidative stress. Antioxidants & Redox Signaling, 23,1144–1170. doi: 10.1089/ars.2015.6317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkins CL, & Davies MJ (2014). Detection and characterisation of radicals in biological materials using EPR methodology. Biochimica et Biophysica Acta-General Subjects, 1840, 708–721. doi: 10.1016/j.bbagen.2013.03.034 [DOI] [PubMed] [Google Scholar]
- Jones DP (2002). Redox potential of GSH/GSSG couple: Assay and biological significance. Methods in Enzymology, 348, 93–112. doi: 10.1016/S00766879(02)48630-2 [DOI] [PubMed] [Google Scholar]
- Jones DP (2008). Radical-free biology of oxidative stress. American Journal of Physiology–Cell Physiology, 295, C849–C868. doi: 10.1152/ajpcell.00283.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lázár R, Orvos H, Szőfiősi R, & Varga IS (2015). The quality of the antioxidant defence system in term and preterm twin neonates. Redox Report, 20, 103–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS (1998). Stress, adaptation, and disease: Allostatsis and allostatic load. Annals of the New York Academy of Sciences, 840, 33–44. doi: 10.1111/j.1749-6632.1998.tb09546.x [DOI] [PubMed] [Google Scholar]
- Moore TA, Ahmad IM, Schmid KK, Berger AM, Ruiz RJ, Pickier RH, & Zimmerman MC (2019). Oxidative stress levels throughout pregnancy, at birth, and in the neonate. Biological Research for Nursing, 21, 485–494. doi: 10.1177/1099800419858670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore TA, Ahmad IM, & Zimmerman MC (2018). Oxidative stress and preterm birth: An integrative review. Biological Research for Nursing, 20, 497–512. doi: 10.1177/1099800418791028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poiettini J, Cobo T, Kacerovsky M, Vinturache AE, Laudanski P, Peeien MJ, … Menon R (2017). Biomarkers of spontaneous preterm birth: A systematic review of studies using multiplex analysis. Journal of Perinatal Medicine, 45, 71–84. doi: 10.1515/jpm-2016-0097 [DOI] [PubMed] [Google Scholar]
- Schieber M, & Chandei NS (2014). ROS function in redox signaling and oxidative stress. Current Biology, 24, R453–R462. doi: 10.1016/j.cub.2014.03.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seis H (2017). Hydrogen peroxide as a central redox signaling molecule in physiological oxidative stress: Oxidative eustress. Redox Biology, 11, 613–619. doi: 10.1016/j.redox.2016.12.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soydinç HE, Sak ME, Evliyaoğlu O, Evsen MS, Turgut A, Özler A, … Gül T (2012). Maternal plasma prolidase, matrix matrixmetalloproteinases 1 and 13, and oxidative stress levels in pregnancies complicated by preterm premature rupture of the membranes and chorioamnionitis. Journal of the Turkish German Gynecological Association, 13, 172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soydinc HE, Sak ME, Evliyaogiu O, Evsen MS, Turgut A, Özier A, … Gui T (2013). Prolidase, matrix metalloproteinases 1 and 13 activity, oxidative-antioxidative status as a marker of preterm premature rupture of membranes and chorioamnionitis in maternal vaginal washing fiuids. International Journal of Medical Sciences, 10, 1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veal EA, Day AM, & Morgan BA (2007). Hydrogen peroxide sensing and signaling. Molecular Cell, 26, 1–14. doi: 10.1016/j.molcel.2007.03.016 [DOI] [PubMed] [Google Scholar]
- Wei SQ, Fraser W, & Luo ZC (2010). Inflammatory cytokines and spontaneous preterm birth in asymptomatic women: A systematic review. Obstetrics & Gynecology, 116, 393–401. doi: 10.1097/AOG.0b013e3181e6dbc0 [DOI] [PubMed] [Google Scholar]


